Transplantation of Mature Adipocyte-Derived Dedifferentiated Fat Cells Facilitates Periodontal Tissue Regeneration of Class II Furcation Defects in Miniature Pigs
- PMID: 35207844
- PMCID: PMC8875781
- DOI: 10.3390/ma15041311
Transplantation of Mature Adipocyte-Derived Dedifferentiated Fat Cells Facilitates Periodontal Tissue Regeneration of Class II Furcation Defects in Miniature Pigs
Abstract
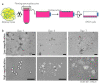
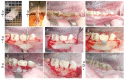
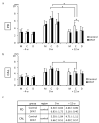
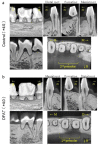
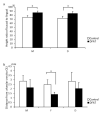
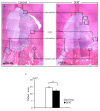
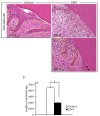
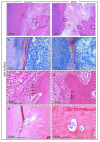
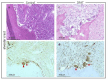
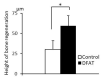
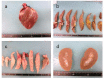
Adipose tissue is composed mostly of adipocytes that are in contact with capillaries. By using a ceiling culture method based on buoyancy, lipid-free fibroblast-like cells, also known as dedifferentiated fat (DFAT) cells, can be separated from mature adipocytes with a large single lipid droplet. DFAT cells can re-establish their active proliferation ability and transdifferentiate into various cell types under appropriate culture conditions. Herein, we sought to compare the regenerative potential of collagen matrix alone (control) with autologous DFAT cell-loaded collagen matrix transplantation in adult miniature pigs (microminipigs; MMPs). We established and transplanted DFAT cells into inflammation-inducing periodontal class II furcation defects. At 12 weeks after cell transplantation, a marked attachment gain was observed based on the clinical parameters of probing depth (PD) and clinical attachment level (CAL). Additionally, micro computed tomography (CT) revealed hard tissue formation in furcation defects of the second premolar. The cemento-enamel junction and alveolar bone crest distance was significantly shorter following transplantation. Moreover, newly formed cellular cementum, well-oriented periodontal ligament-like fibers, and alveolar bone formation were observed via histological analysis. No teratomas were found in the internal organs of recipient MMPs. Taken together, these findings suggest that DFAT cells can safely enhance periodontal tissue regeneration.
Keywords: dedifferentiated fat cells; periodontal furcation defect; periodontal tissue regeneration; transplantation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Regeneration of Two-Walled Infrabony Periodontal Defects in Swine After Buccal Fat Pad-Derived Dedifferentiated Fat Cell Autologous Transplantation.Biomolecules. 2025 Apr 20;15(4):604. doi: 10.3390/biom15040604. Biomolecules. 2025. PMID: 40305349 Free PMC article.
-
Use of Rat Mature Adipocyte-Derived Dedifferentiated Fat Cells as a Cell Source for Periodontal Tissue Regeneration.Front Physiol. 2016 Feb 23;7:50. doi: 10.3389/fphys.2016.00050. eCollection 2016. Front Physiol. 2016. PMID: 26941649 Free PMC article.
-
Transplantation of mature adipocyte-derived dedifferentiated fat cells into three-wall defects in the rat periodontium induces tissue regeneration.J Oral Sci. 2017;59(4):611-620. doi: 10.2334/josnusd.16-0878. J Oral Sci. 2017. PMID: 29279571
-
Histologic evidence of periodontal regeneration in furcation defects: a systematic review.Clin Oral Investig. 2019 Jul;23(7):2861-2906. doi: 10.1007/s00784-019-02964-3. Epub 2019 Jun 4. Clin Oral Investig. 2019. PMID: 31165313
-
Biological aspects of periodontal tissue regeneration: cementogenesis and the induction of Sharpey's fibres.SADJ. 2013 Aug;68(7):304-6, 308-12, 314 passim. SADJ. 2013. PMID: 24133950 Review.
Cited by
-
Collagen Scaffolds Laden with Human Periodontal Ligament Fibroblasts Promote Periodontal Regeneration in SD Rat Model.Polymers (Basel). 2023 Jun 12;15(12):2649. doi: 10.3390/polym15122649. Polymers (Basel). 2023. PMID: 37376295 Free PMC article.
-
Adipose Tissue-Derived Therapies for Osteoarthritis: Multifaceted Mechanisms and Clinical Prospects.Cells. 2025 May 2;14(9):669. doi: 10.3390/cells14090669. Cells. 2025. PMID: 40358193 Free PMC article. Review.
-
Regeneration of Two-Walled Infrabony Periodontal Defects in Swine After Buccal Fat Pad-Derived Dedifferentiated Fat Cell Autologous Transplantation.Biomolecules. 2025 Apr 20;15(4):604. doi: 10.3390/biom15040604. Biomolecules. 2025. PMID: 40305349 Free PMC article.
References
LinkOut - more resources
Full Text Sources

