The Advances and Challenges of Liposome-Assisted Drug Release in the Presence of Serum Albumin Molecules: The Influence of Surrounding pH
- PMID: 35208126
- PMCID: PMC8874444
- DOI: 10.3390/ma15041586
The Advances and Challenges of Liposome-Assisted Drug Release in the Presence of Serum Albumin Molecules: The Influence of Surrounding pH
Abstract
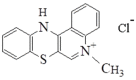
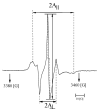
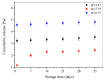
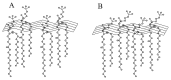
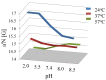
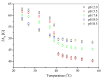
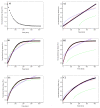
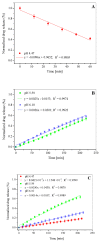
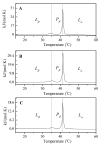
The aim of this study is to prepare a liposomal delivery system for 5-methyl-12 (H)-quino[3,4-b]-1,4-benzothiazine chloride (5-MBT) and study the in vitro release characteristics. The release of 5-MBT from a liposomal complex with human serum albumin (HSA) [LDPPC/5-MBT]:HSA was examined using the spectrophotometric method and differential scanning calorimetry (DSC). Electronic paramagnetic resonance was used to assess the influence of the pH of the environment on the conformation of phospholipids, the latter determining the degree of release of the encapsulated compound. The applied mathematical models made it possible to determine the necessary analytical parameters to facilitate the process of potential drug release from liposomes. The complexes formed by liposomal 5-MBT with serum albumin (HSA) particles allowed for the description of the Fick process. The change in the polarity of the phospholipid membrane resulting from the changes in the pH of the surroundings, significantly influenced the percentage of 5-MBT entrapment in the liposomes. It also affected the release percentage.
Keywords: biological systems; controlled drug delivery; nanoparticles; release mechanism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources

