Photothermal and Photodynamic Therapy of Tumors with Plasmonic Nanoparticles: Challenges and Prospects
- PMID: 35208145
- PMCID: PMC8878601
- DOI: 10.3390/ma15041606
Photothermal and Photodynamic Therapy of Tumors with Plasmonic Nanoparticles: Challenges and Prospects
Abstract
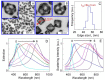
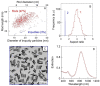
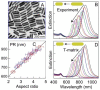
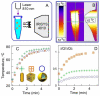
Cancer remains one of the leading causes of death in the world. For a number of neoplasms, the efficiency of conventional chemo- and radiation therapies is insufficient because of drug resistance and marked toxicity. Plasmonic photothermal therapy (PPT) using local hyperthermia induced by gold nanoparticles (AuNPs) has recently been extensively explored in tumor treatment. However, despite attractive promises, the current PPT status is limited by laboratory experiments, academic papers, and only a few preclinical studies. Unfortunately, most nanoformulations still share a similar fate: great laboratory promises and fair preclinical trials. This review discusses the current challenges and prospects of plasmonic nanomedicine based on PPT and photodynamic therapy (PDT). We start with consideration of the fundamental principles underlying plasmonic properties of AuNPs to tune their plasmon resonance for the desired NIR-I, NIR-2, and SWIR optical windows. The basic principles for simulation of optical cross-sections and plasmonic heating under CW and pulsed irradiation are discussed. Then, we consider the state-of-the-art methods for wet chemical synthesis of the most popular PPPT AuNPs such as silica/gold nanoshells, Au nanostars, nanorods, and nanocages. The photothermal efficiencies of these nanoparticles are compared, and their applications to current nanomedicine are shortly discussed. In a separate section, we discuss the fabrication of gold and other nanoparticles by the pulsed laser ablation in liquid method. The second part of the review is devoted to our recent experimental results on laser-activated interaction of AuNPs with tumor and healthy tissues and current achievements of other research groups in this application area. The unresolved issues of PPT are the significant accumulation of AuNPs in the organs of the mononuclear phagocyte system, causing potential toxic effects of nanoparticles, and the possibility of tumor recurrence due to the presence of survived tumor cells. The prospective ways of solving these problems are discussed, including developing combined antitumor therapy based on combined PPT and PDT. In the conclusion section, we summarize the most urgent needs of current PPT-based nanomedicine.
Keywords: gold nanoparticles; oncology; photodynamic therapy; plasmonic photothermal therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Towards Effective Photothermal/Photodynamic Treatment Using Plasmonic Gold Nanoparticles.Int J Mol Sci. 2016 Aug 9;17(8):1295. doi: 10.3390/ijms17081295. Int J Mol Sci. 2016. PMID: 27517913 Free PMC article. Review.
-
Exploiting gold nanoparticles for diagnosis and cancer treatments.Nanotechnology. 2021 May 7;32(19):192001. doi: 10.1088/1361-6528/abe1ed. Nanotechnology. 2021. PMID: 33524960 Review.
-
Galvanic replacement synthesis of multi-branched gold nanocrystals for photothermal cancer therapy.J Mater Chem B. 2020 Jul 1;8(25):5491-5499. doi: 10.1039/d0tb00748j. J Mater Chem B. 2020. PMID: 32478780
-
Nanoscaled PAMAM Dendrimer Spacer Improved the Photothermal-Photodynamic Treatment Efficiency of Photosensitizer-Decorated Confeito-Like Gold Nanoparticles for Cancer Therapy.Macromol Biosci. 2022 Aug;22(8):e2200130. doi: 10.1002/mabi.202200130. Epub 2022 May 24. Macromol Biosci. 2022. PMID: 35579182
-
Plasmonic photothermal therapy: Approaches to advanced strategy.Lasers Surg Med. 2018 Dec;50(10):1025-1033. doi: 10.1002/lsm.23001. Epub 2018 Jul 19. Lasers Surg Med. 2018. PMID: 30024039
Cited by
-
Photodynamic Therapy for Inflammatory and Cancerous Diseases of the Intestines: Molecular Mechanisms and Prospects for Application.Int J Biol Sci. 2023 Sep 4;19(15):4793-4810. doi: 10.7150/ijbs.87492. eCollection 2023. Int J Biol Sci. 2023. PMID: 37781521 Free PMC article. Review.
-
The Investigation of the Chemical Composition and Applicability of Gold Nanoparticles Synthesized with Amygdalus communis (Almond) Leaf Aqueous Extract as Antimicrobial and Anticancer Agents.Molecules. 2023 Mar 7;28(6):2428. doi: 10.3390/molecules28062428. Molecules. 2023. PMID: 36985400 Free PMC article.
-
Nanomedicines Targeting Metabolic Pathways in the Tumor Microenvironment: Future Perspectives and the Role of AI.Metabolites. 2025 Mar 13;15(3):201. doi: 10.3390/metabo15030201. Metabolites. 2025. PMID: 40137165 Free PMC article. Review.
-
pH-Dependent HEWL-AuNPs Interactions: Optical Study.Molecules. 2023 Dec 22;29(1):82. doi: 10.3390/molecules29010082. Molecules. 2023. PMID: 38202662 Free PMC article.
-
Smart Nanostructured Materials for SARS-CoV-2 and Variants Prevention, Biosensing and Vaccination.Biosensors (Basel). 2022 Dec 5;12(12):1129. doi: 10.3390/bios12121129. Biosensors (Basel). 2022. PMID: 36551096 Free PMC article. Review.
References
-
- Bohren C.F., Huffman D.R. Absorption and Scattering of Light by Small Particles. John and Wiley and Sons; Hoboken, NJ, USA: 2008.
-
- Khlebtsov N., Dykman L.A. Optical Properties and Biomedical Applications of Plasmonic Nanoparticles. J. Quant. Spectrosc. Radiat. Transf. 2010;111:1–35. doi: 10.1016/j.jqsrt.2009.07.012. - DOI
-
- Khlebtsov N.G. T-Matrix Method in Plasmonics: An Overview. J. Quant. Spectrosc. Radiat. Transf. 2013;123:184–217. doi: 10.1016/j.jqsrt.2012.12.027. - DOI
-
- Klimov V. Nanoplasmonics. CRC Press; Boca Raton, FL, USA: 2014. - DOI
Publication types
Grants and funding
- 18-14-00016-П/Russian Science Foundation
- 20-52-56005/Russian Foundation for Basic Research
- 19-32-90224/Russian Foundation for Basic Research
- 15929GU/2020/The Fund for Promoting Innovation grant UMNIK-19 / HealthNet-NTI - 2019
- 075-15-2021-615/Decree of the Government of the Russian Federation No. 220 of 09 April 2010
LinkOut - more resources
Full Text Sources
Miscellaneous

