Molecular Mechanisms of Parathyroid Disorders in Chronic Kidney Disease
- PMID: 35208186
- PMCID: PMC8878033
- DOI: 10.3390/metabo12020111
Molecular Mechanisms of Parathyroid Disorders in Chronic Kidney Disease
Abstract
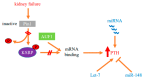
Secondary hyperparathyroidism (SHP) is a common complication of chronic kidney disease (CKD) that induces morbidity and mortality in patients. How CKD stimulates the parathyroid to increase parathyroid hormone (PTH) secretion, gene expression and cell proliferation remains an open question. In experimental SHP, the increased PTH gene expression is post-transcriptional and mediated by PTH mRNA-protein interactions that promote PTH mRNA stability. These interactions are orchestrated by the isomerase Pin1. Pin1 participates in conformational change-based regulation of target proteins, including mRNA-binding proteins. In SHP, Pin1 isomerase activity is decreased, and thus, the Pin1 target and PTH mRNA destabilizing protein KSRP fails to bind PTH mRNA, increasing PTH mRNA stability and levels. An additional level of post-transcriptional regulation is mediated by microRNA (miRNA). Mice with parathyroid-specific knockout of Dicer, which facilitates the final step in miRNA maturation, lack parathyroid miRNAs but have normal PTH and calcium levels. Surprisingly, these mice fail to increase serum PTH in response to hypocalcemia or uremia, indicating a role for miRNAs in parathyroid stimulation. SHP often leads to parathyroid hyperplasia. Reduced expressions of parathyroid regulating receptors, activation of transforming growth factor α-epidermal growth factor receptor, cyclooxygenase 2-prostaglandin E2 and mTOR signaling all contribute to the enhanced parathyroid cell proliferation. Inhibition of mTOR by rapamycin prevents and corrects the increased parathyroid cell proliferation of SHP. This review summarizes the current knowledge on the mechanisms that stimulate the parathyroid cell at multiple levels in SHP.
Keywords: microRNA; parathyroid cell proliferation; parathyroid hormone; post-transcriptional regulation; secondary hyperparathyroidism.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Silver J., Levi R. Regulation of pth synthesis and secretion relevant to the management of secondary hyperparathyroidism in chronic kidney disease. [(accessed on 20 December 2021)];Kidney Int. Suppl. 2005 67:S8–S12. doi: 10.1111/j.1523-1755.2005.09501.x. Available online: http://www.ncbi.nlm.nih.gov/pubmed/15882315. - DOI - PubMed
-
- Kumar R., Thompson J.R. The regulation of parathyroid hormone secretion and synthesis. [(accessed on 20 December 2021)];J. Am. Soc. Nephrol. 2011 22:216–224. doi: 10.1681/ASN.2010020186. Available online: https://pubmed.ncbi.nlm.nih.gov/21164021. - DOI - PMC - PubMed
-
- Hofer M.A., Brown E.M. Extracellular calcium sensing and signalling. [(accessed on 20 December 2021)];Nat. Rev. Mol. Cell Biol. 2003 4:530–538. doi: 10.1038/nrm1154. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12838336. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

