Assessment of Physiological Rat Kidney Ageing-Implications for the Evaluation of Allograft Quality Prior to Renal Transplantation
- PMID: 35208236
- PMCID: PMC8875225
- DOI: 10.3390/metabo12020162
Assessment of Physiological Rat Kidney Ageing-Implications for the Evaluation of Allograft Quality Prior to Renal Transplantation
Abstract
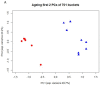
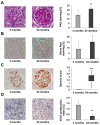
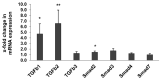
Due to organ shortage and rising life expectancy the age of organ donors and recipients is increasing. Reliable biomarkers of organ quality that predict successful long-term transplantation outcomes are poorly defined. The aim of this study was the identification of age-related markers of kidney function that might accurately reflect donor organ quality. Histomorphometric, biochemical and molecular parameters were measured in young (3-month-old) and old (24-month-old) male Sprague Dawley rats. In addition to conventional methods, we used urine metabolomics by NMR spectroscopy and gene expression analysis by quantitative RT-PCR to identify markers of ageing relevant to allograft survival. Beside known markers of kidney ageing like albuminuria, changes in the concentration of urine metabolites such as trimethylamine-N-oxide, trigonelline, 2-oxoglutarate, citrate, hippurate, glutamine, acetoacetate, valine and 1-methyl-histidine were identified in association with ageing. In addition, expression of several genes of the toll-like receptor (TLR) pathway, known for their implication in inflammaging, were upregulated in the kidneys of old rats. This study led to the identification of age-related markers of biological allograft age potentially relevant for allograft survival in the future. Among those, urine metabolites and markers of immunity and inflammation, which are highly relevant to immunosuppression in transplant recipients, are promising and deserve further investigation in humans.
Keywords: ageing; inflammation; kidney; transplantation; urinary metabolites.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- [(accessed on 15 October 2021)]. Available online: https://www.eurotransplant.org/organs/kidney/
Grants and funding
LinkOut - more resources
Full Text Sources

