Protein Dielectrophoresis: A Tale of Two Clausius-Mossottis-Or Something Else?
- PMID: 35208384
- PMCID: PMC8876334
- DOI: 10.3390/mi13020261
Protein Dielectrophoresis: A Tale of Two Clausius-Mossottis-Or Something Else?
Abstract
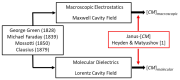
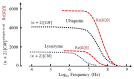
Standard DEP theory, based on the Clausius-Mossotti (CM) factor derived from solving the boundary-value problem of macroscopic electrostatics, fails to describe the dielectrophoresis (DEP) data obtained for 22 different globular proteins over the past three decades. The calculated DEP force appears far too small to overcome the dispersive forces associated with Brownian motion. An empirical theory, employing the equivalent of a molecular version of the macroscopic CM-factor, predicts a protein's DEP response from the magnitude of the dielectric β-dispersion produced by its relaxing permanent dipole moment. A new theory, supported by molecular dynamics simulations, replaces the macroscopic boundary-value problem with calculation of the cross-correlation between the protein and water dipoles of its hydration shell. The empirical and formal theory predicts a positive DEP response for protein molecules up to MHz frequencies, a result consistently reported by electrode-based (eDEP) experiments. However, insulator-based (iDEP) experiments have reported negative DEP responses. This could result from crystallization or aggregation of the proteins (for which standard DEP theory predicts negative DEP) or the dominating influences of electrothermal and other electrokinetic (some non-linear) forces now being considered in iDEP theory.
Keywords: Clausius–Mossotti function; Lorentz cavity; Maxwell cavity; dielectric beta-dispersion; dielectrophoresis; electrokinetics; molecular dynamics simulations; proteins.
Conflict of interest statement
The author declares no conflict of interest.
Figures









Similar articles
-
Protein Dielectrophoresis: I. Status of Experiments and an Empirical Theory.Micromachines (Basel). 2020 May 22;11(5):533. doi: 10.3390/mi11050533. Micromachines (Basel). 2020. PMID: 32456059 Free PMC article.
-
Protein dielectrophoresis: Key dielectric parameters and evolving theory.Electrophoresis. 2021 Mar;42(5):513-538. doi: 10.1002/elps.202000255. Epub 2020 Nov 30. Electrophoresis. 2021. PMID: 33084076 Review.
-
Protein Dielectrophoresis in Solution.J Phys Chem B. 2018 Oct 4;122(39):9119-9127. doi: 10.1021/acs.jpcb.8b06864. Epub 2018 Sep 25. J Phys Chem B. 2018. PMID: 30205677
-
Limitations of the Clausius-Mossotti function used in dielectrophoresis and electrical impedance studies of biomacromolecules.Electrophoresis. 2019 Sep;40(18-19):2575-2583. doi: 10.1002/elps.201900057. Epub 2019 Mar 19. Electrophoresis. 2019. PMID: 30861572
-
Particle trapping in electrically driven insulator-based microfluidics: Dielectrophoresis and induced-charge electrokinetics.Electrophoresis. 2021 Dec;42(23):2445-2464. doi: 10.1002/elps.202100123. Epub 2021 Jun 15. Electrophoresis. 2021. PMID: 34081787 Free PMC article. Review.
Cited by
-
Dielectrophoresis from the System's Point of View: A Tale of Inhomogeneous Object Polarization, Mirror Charges, High Repelling and Snap-to-Surface Forces and Complex Trajectories Featuring Bifurcation Points and Watersheds.Micromachines (Basel). 2022 Jun 26;13(7):1002. doi: 10.3390/mi13071002. Micromachines (Basel). 2022. PMID: 35888819 Free PMC article.
-
Editorial for the Special Issue on Micromachines for Dielectrophoresis, Volume II.Micromachines (Basel). 2023 Mar 30;14(4):769. doi: 10.3390/mi14040769. Micromachines (Basel). 2023. PMID: 37421002 Free PMC article.
-
Charged Biological Membranes Repel Large Neutral Molecules by Surface Dielectrophoresis and Counterion Pressure.J Am Chem Soc. 2024 Jan 31;146(4):2701-2710. doi: 10.1021/jacs.3c12348. Epub 2024 Jan 16. J Am Chem Soc. 2024. PMID: 38291994 Free PMC article.
-
Editorial for the Micro/Nanoscale Electrokinetics Section.Micromachines (Basel). 2024 Nov 25;15(12):1414. doi: 10.3390/mi15121414. Micromachines (Basel). 2024. PMID: 39770167 Free PMC article.
-
Dielectrophoresis: An Approach to Increase Sensitivity, Reduce Response Time and to Suppress Nonspecific Binding in Biosensors?Biosensors (Basel). 2022 Sep 23;12(10):784. doi: 10.3390/bios12100784. Biosensors (Basel). 2022. PMID: 36290922 Free PMC article. Review.
References
-
- Washizu M., Suzuki S., Kurosawa O., Nishizaka T., Shinohara T. Molecular dielectrophoresis of biopolymers. IEEE Trans. Ind. Appl. 1994;30:835–843. doi: 10.1109/28.297897. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

