Fibroblast Derived Skin Wound Healing Modeling on Chip under the Influence of Micro-Capillary Shear Stress
- PMID: 35208429
- PMCID: PMC8876720
- DOI: 10.3390/mi13020305
Fibroblast Derived Skin Wound Healing Modeling on Chip under the Influence of Micro-Capillary Shear Stress
Abstract
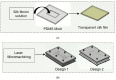
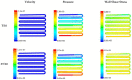
Fibroblast cell migration plays a crucial role in the wound-healing process. Hence, its quantitative investigation is important to understand the mechanism of the wound-healing process. The dynamic nature of the wound-healing process can be easily implemented using a microfluidic-based wound-healing assay. This work presented the use of a microfluidics device to simulate traumatic wounds on fibroblast cell monolayers by utilizing trypsin flow and PDMS barrier. In this study, a microfluidic chip with a transparent silk film is reported. The placement of film provides 3D cell culture conditions that mimic a 3D extracellular matrix (ECM) like environment and allows real-time monitoring of cells. A numerical study was conducted to evaluate the influence of dynamic medium-induced shear stress on the base and wall of the microchannel. This could facilitate the optimization of the inlet flow conditions of the media in the channel. At the same time, it could help in identifying stress spots in the channel. The scaffolds were placed in those spots for evaluating the influence of shear forces on the migratory behavior of fibroblast cells. The in vitro microfluidic assembly was then evaluated for cell migration under the influence of external shear forces during the wound-healing phenomena. A faster wound healing was obtained at the end of 24 h of the creation of the wound in the presence of optimal shear stress. On increasing the shear stress beyond a threshold limit, it dissociates fibroblast cells from the surface of the substrate, thereby decelerating the wound-healing process. The above phenomena were transformed in both coplanar microfluidics surfaces (by realizing in the multichannel interlinked model) and transitional microfluidics channels (by realizing in the sandwich model).
Keywords: fibroblast; microchannels; multilayered channels; sandwich model; shear stress; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













References
-
- Bainbridge P. Wound healing and the role of fibroblasts. J. Wound Care. 2013;22:407–411. - PubMed
LinkOut - more resources
Full Text Sources

