Morphological Phenotypes, Cell Division, and Gene Expression of Escherichia coli under High Concentration of Sodium Sulfate
- PMID: 35208727
- PMCID: PMC8875244
- DOI: 10.3390/microorganisms10020274
Morphological Phenotypes, Cell Division, and Gene Expression of Escherichia coli under High Concentration of Sodium Sulfate
Abstract
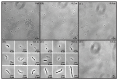
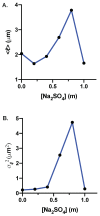
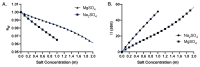
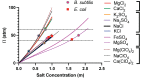
Sodium and sulfate ions are among the suggested abundant ions on Europa, a moon of Jupiter. In order to investigate the potential habitability of Europa, we study the effects of sodium sulfate (Na2SO4) on a non-halophilic bacterium by subjecting Escherichia coli (E. coli) to a wide range of Na2SO4 concentrations (0-1.0 m). We discover that, as the concentration of sodium sulfate increases, the biomass doubling time increases and the cell growth is completely inhibited at 1.0 m Na2SO4. Furthermore, we find that E. coli exhibits three distinct morphological phenotypes-(i) shortened, (ii) normal, and (iii) elongated/filamented cells at 0.6 m and 0.8 m Na2SO4. We have examined the expression of different genes involved in sodium and sulfate transport (nhaA, nhaB, cysZ, sbp), osmotically driven transport of water (aqpZ), sulfate metabolism (cysN), fatty acid production (fabA), and a global transcriptional regulator (osmZ). Our results suggest that the expression of these genes is not affected significantly at high concentrations of sodium sulfate in the exponential growth phase. Using our experimental data and the existing data in the literature, we show that the osmotic pressure difference may play a major role in determining the growth inhibition of E. coli and B. subtilis at high concentrations of salt.
Keywords: cellular response of Escherichia coli under hyperosmolar stress; habitability of Europa; sodium sulfate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Kato C., Sato T., Horikoshi K. Isolation and properties of barophilic and barotolerant bacteria from deep-sea mud samples. Biodivers. Conserv. 1995;4:1–9. doi: 10.1007/BF00115311. - DOI
-
- Antón J., Oren A., Benlloch S., Rodríguez-Valera F., Amann R., Rosselló-Mora R. Salinibacter ruber gen. nov., sp. nov., a novel, extremely halophilic member of the Bacteria from saltern crystallizer ponds. Int. J. Syst. Evol. Microbiol. 2002;52:485–491. doi: 10.1099/00207713-52-2-485. - DOI - PubMed
LinkOut - more resources
Full Text Sources

