Differences in UV-C LED Inactivation of Legionellapneumophila Serogroups in Drinking Water
- PMID: 35208810
- PMCID: PMC8877565
- DOI: 10.3390/microorganisms10020352
Differences in UV-C LED Inactivation of Legionellapneumophila Serogroups in Drinking Water
Abstract
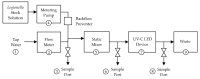
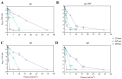
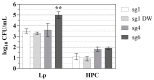
Legionella pneumophila (Lp) is an opportunistic pathogen that causes respiratory infections primarily through inhalation of contaminated aerosols. Lp can colonize premise plumbing systems due to favorable growth conditions (e.g., lower disinfectant residual, stagnation, warm temperatures). UV-C light-emitting diodes (UV-C LEDs) are an emerging water treatment technology and have been shown to effectively inactivate waterborne pathogens. In this study, the inactivation of four Lp strains (three clinical sg1, 4, and 6; and one sg1 drinking water (DW) isolate) was evaluated using a UV-C LED collimated beam at three wavelengths (255, 265, and 280 nm) and six fluence rates (0.5-34 mJ/cm2). Exposure to 255 nm resulted in higher log reductions at the lower fluences compared to exposures at 265 and 280 nm. Efficacy testing was also performed using a UV-C LED point-of-entry (POE) flow-through device. Based on the log inactivation curves, at 255 nm, the sg4 and sg6 clinical isolates were more susceptible to inactivation compared to the two sg1 isolates. However, at 265 and 280 nm, the sg1 and sg4 clinical isolates were more resistant to inactivation compared to the sg6 clinical and sg1 DW isolates. Differential log reductions were also observed using the POE device. Results indicate that although UV-C LED disinfection is effective, variations in Lp inactivation, wavelengths, and technology applications should be considered, especially when targeting specific isolates within premise plumbing systems.
Keywords: UV-C LED disinfection; decontamination; mechanisms; opportunistic pathogens; potable water; premise plumbing; treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Fields B.S. Legionellae. In: Bitton G., editor. Encyclopedia of Environmental Microbiology. Wiley; Hoboken, NJ, USA: 2003. pp. 1788–1796.
-
- Garrison L.E., Kunz J.M., Cooley L.A., Moore M.R., Lucas C., Schrag S., Sarisky J., Whitney C.G. Vital Signs: Deficiencies in Environmental Control Identified in Outbreaks of Legionnaires’ Disease—North America, 2000–2014. MMWR Morb. Mortal. Wkly. Rep. 2016;65:576–584. doi: 10.15585/mmwr.mm6522e1. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

