Membrane Efflux Pumps of Pathogenic Vibrio Species: Role in Antimicrobial Resistance and Virulence
- PMID: 35208837
- PMCID: PMC8875612
- DOI: 10.3390/microorganisms10020382
Membrane Efflux Pumps of Pathogenic Vibrio Species: Role in Antimicrobial Resistance and Virulence
Abstract
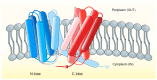
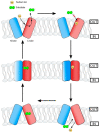
Infectious diseases caused by bacterial species of the Vibrio genus have had considerable significance upon human health for centuries. V. cholerae is the causative microbial agent of cholera, a severe ailment characterized by profuse watery diarrhea, a condition associated with epidemics, and seven great historical pandemics. V. parahaemolyticus causes wound infection and watery diarrhea, while V. vulnificus can cause wound infections and septicemia. Species of the Vibrio genus with resistance to multiple antimicrobials have been a significant health concern for several decades. Mechanisms of antimicrobial resistance machinery in Vibrio spp. include biofilm formation, drug inactivation, target protection, antimicrobial permeability reduction, and active antimicrobial efflux. Integral membrane-bound active antimicrobial efflux pump systems include primary and secondary transporters, members of which belong to closely related protein superfamilies. The RND (resistance-nodulation-division) pumps, the MFS (major facilitator superfamily) transporters, and the ABC superfamily of efflux pumps constitute significant drug transporters for investigation. In this review, we explore these antimicrobial transport systems in the context of Vibrio spp. pathogenesis and virulence.
Keywords: antimicrobial resistance; bacteria; cholera; infection; multidrug efflux pump; multidrug resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- CDC Cholera—Vibrio cholerae infection|Cholera|CDC. [(accessed on 10 October 2019)]; Available online: https://www.cdc.gov/cholera/index.html.
-
- Bonnin-Jusserand M., Copin S., Le Bris C., Brauge T., Gay M., Brisabois A., Grard T., Midelet-Bourdin G. Vibrio species involved in seafood-borne outbreaks (Vibrio cholerae, V. parahaemolyticus and V. vulnificus): Review of microbiological versus recent molecular detection methods in seafood products. Crit. Rev. Food Sci. Nutr. 2019;59:597–610. doi: 10.1080/10408398.2017.1384715. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

