Three-Week Old Pigs Are Not Susceptible to Productive Infection with SARS-COV-2
- PMID: 35208863
- PMCID: PMC8875799
- DOI: 10.3390/microorganisms10020407
Three-Week Old Pigs Are Not Susceptible to Productive Infection with SARS-COV-2
Abstract
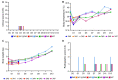
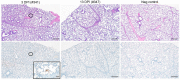
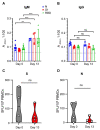
As the COVID-19 pandemic moves into its third year, there remains a need for additional animal models better recapitulating severe COVID to study SARS-CoV-2 pathogenesis and develop countermeasures, especially treatment options. Pigs are known intermediate hosts for many viruses with zoonotic potential and are susceptible to infection with alpha, beta and delta genera of coronaviruses. Herein, we infected young (3 weeks of age) pigs with SARS-CoV-2 using a combination of respiratory and parenteral inoculation routes. Pigs did not develop clinical disease, nor macroscopic or microscopic pathologic lesions upon SARS-CoV-2 infection. Despite occasional low levels of SARS-CoV-2 genomic RNA in the respiratory tract, subgenomic RNA and infectious virus were never found, and SARS-CoV-2-specific adaptive immune responses were not detectable over the 13-day study period. We concluded that pigs are not susceptible to productive SARS-CoV-2 infection and do not serve as a SARS-CoV-2 reservoir for zoonotic transmission.
Keywords: SARS-CoV-2; disease; infection; pathology; replication; transmission; young pigs.
Conflict of interest statement
J.A.R. received support from Tonix Pharmaceuticals, Xing Technologies and Zoetis, outside of the reported work. J.A.R. is an inventor of patents and patent applications on the use of antivirals and vaccines for the treatment and prevention of virus infections, owned by Kansas State University. All other authors declare no conflict of interest.
Figures
References
-
- World Health Organization . WHO Coronavirus (COVID-19) Dashboard. WHO; Geneva, Switzerland: 2021. [(accessed on 20 December 2021)]. Available online: https://covid19.who.int/
-
- World Health Organization Tracking SARS-CoV-2 Variants. [(accessed on 20 December 2021)]. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

