Vaccination against Tick-Borne Encephalitis (TBE) in Italy: Still a Long Way to Go
- PMID: 35208918
- PMCID: PMC8880353
- DOI: 10.3390/microorganisms10020464
Vaccination against Tick-Borne Encephalitis (TBE) in Italy: Still a Long Way to Go
Abstract
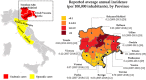
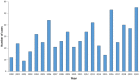
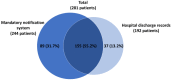
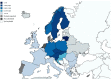
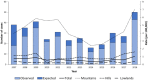
Tick-borne encephalitis (TBE) is endemic in several European countries, and its incidence has recently increased. Various factors may explain this phenomenon: social factors (changes in human behavior, duration and type of leisure activities and increased tourism in European high-risk areas), ecological factors (e.g., effects of climate change on the tick population and reservoir animals), and technological factors (improved diagnostics, increased medical awareness). Furthermore, the real burden of TBE is not completely known, as the performance of surveillance systems is suboptimal and cases of disease are under-reported in several areas. Given the potentially severe clinical course of the disease, the absence of any antiviral therapy, and the impossibility of interrupting the transmission of the virus in nature, vaccination is the mainstay of prevention and control. TBE vaccines are effective (protective effect of approximately 95% after completion of the basic vaccination-three doses) and well tolerated. However, their uptake in endemic areas is suboptimal. In the main endemic countries where vaccination is included in the national/regional immunization program (with reimbursed vaccination programs), this decision was driven by a cost-effectiveness assessment (CEA), which is a helpful tool in the decision-making process. All CEA studies conducted have demonstrated the cost-effectiveness of TBE vaccination. Unfortunately, CEA is still lacking in many endemic countries, including Italy. In the future, it will be necessary to fill this gap in order to introduce an effective vaccination strategy in endemic areas. Finally, raising awareness of TBE, its consequences and the benefit of vaccination is critical in order to increase vaccination coverage and reduce the burden of the disease.
Keywords: TBE; TBE vaccine; cost-effectiveness assessment; tick-borne encephalitis; tick-borne encephalitis virus; vaccination; vaccination strategies.
Conflict of interest statement
The authors declare the following financial interests/personal relationships, which may be regarded as potential competing interests: R.I. and P.R. are employees of Pfizer and may hold Pfizer stock or stock options. D.P. received funding/support from Pfizer. D.A. and A.D. have no conflicts of interests regarding this manuscript.
Figures
References
-
- Domnich A., Panatto D., Arbuzova E.K., Signori A., Avio U., Gasparini R., Amicizia D. Immunogenicity against Far Eastern and Siberian Subtypes of Tick-borne Encephalitis (TBE) Virus Elicited by the Currently Available Vaccines Based on the European Subtype: Systematic Review and Meta-Analysis. Hum. Vaccines Immunother. 2014;10:2819–2833. doi: 10.4161/hv.29984. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

