Part II: NiMoO4 Nanostructures Synthesized by the Solution Combustion Method: A Parametric Study on the Influence of Material Synthesis and Electrode-Fabrication Parameters on the Electrocatalytic Activity in the Hydrogen Evolution Reaction
- PMID: 35208991
- PMCID: PMC8876296
- DOI: 10.3390/molecules27041199
Part II: NiMoO4 Nanostructures Synthesized by the Solution Combustion Method: A Parametric Study on the Influence of Material Synthesis and Electrode-Fabrication Parameters on the Electrocatalytic Activity in the Hydrogen Evolution Reaction
Abstract
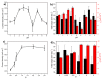
Earth-abundant NiMo-oxide nanostructures were investigated as efficient electrocatalytic materials for the hydrogen evolution reaction (HER) in acidic media. Synthesis and non-synthesis parameters were thoroughly studied. For the non-synthesis parameters, the variation in Nafion loading resulted in a volcano-like trend, while the change in the electrocatalyst loading showed that the marginal benefit of high loadings attenuates due to mass-transfer limitations. The addition of carbon black to the electrocatalyst layer improved the HER performance at low loadings. Different carbon black grades showed a varying influence on the HER performance. Regarding the synthesis parameters, a calcination temperature of 500 °C, a calcination time between 20 and 720 min, a stoichiometric composition (Ni/Mo = 1), an acidic precursor solution, and a fuel-lean system were conditions that yielded the highest HER activity. The in-house NiMoO4/CB/Nafion electrocatalyst layer was found to offer a better long-term performance than the commercial Pt/C.
Keywords: hydrogen evolution reaction; nanostructures; nickel molybdate; parametric study; solution combustion synthesis; water electrolysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Ursua A., Gandia L.M., Sanchis P. Hydrogen production from water electrolysis: Current status and future trends. Proc. IEEE. 2011;100:410–426. doi: 10.1109/JPROC.2011.2156750. - DOI
-
- Wang M., Wang Z., Gong X., Guo Z. The intensification technologies to water electrolysis for hydrogen production—A review. Renew. Sustain. Energy Rev. 2014;29:573–588. doi: 10.1016/j.rser.2013.08.090. - DOI
-
- Dyer C.K. Fuel cells for portable applications. J. Power Sources. 2002;106:31–34. doi: 10.1016/S0378-7753(01)01069-2. - DOI
-
- Mazloomi K., Gomes C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012;16:3024–3033. doi: 10.1016/j.rser.2012.02.028. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

