Induction of Liver Size Reduction in Zebrafish Larvae by the Emerging Synthetic Cannabinoid 4F-MDMB-BINACA and Its Impact on Drug Metabolism
- PMID: 35209079
- PMCID: PMC8879502
- DOI: 10.3390/molecules27041290
Induction of Liver Size Reduction in Zebrafish Larvae by the Emerging Synthetic Cannabinoid 4F-MDMB-BINACA and Its Impact on Drug Metabolism
Abstract
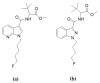
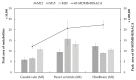
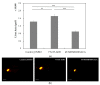
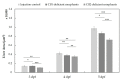
Zebrafish (ZF; Danio rerio) larvae have become a popular in vivo model in drug metabolism studies. Here, we investigated the metabolism of methyl 2-[1-(4-fluorobutyl)-1H-indazole-3-carboxamido]-3,3-dimethylbutanoate (4F-MDMB-BINACA) in ZF larvae after direct administration of the cannabinoid via microinjection, and we visualized the spatial distributions of the parent compound and its metabolites by mass spectrometry imaging (MSI). Furthermore, using genetically modified ZF larvae, the role of cannabinoid receptor type 1 (CB1) and type 2 (CB2) on drug metabolism was studied. Receptor-deficient ZF mutant larvae were created using morpholino oligonucleotides (MOs), and CB2-deficiency had a critical impact on liver development of ZF larva, leading to a significant reduction of liver size. A similar phenotype was observed when treating wild-type ZF larvae with 4F-MDMB-BINACA. Thus, we reasoned that the cannabinoid-induced impaired liver development might also influence its metabolic function. Studying the metabolism of two synthetic cannabinoids, 4F-MDMB-BINACA and methyl 2-(1-(5-fluoropentyl)-1H-pyrrolo[2,3-b]pyridine-3-carboxamido)-3,3-dimethylbutanoate (7'N-5F-ADB), revealed important insights into the in vivo metabolism of these compounds and the role of cannabinoid receptor binding.
Keywords: cannabinoid receptors (CB1 and CB2); drug metabolism; hepatotoxicity; mass spectrometry imaging (MSI); methyl 2-(1-(5-fluoropentyl)-1H-pyrrolo[2,3-b]pyridine-3-carboxamido)-3,3-dimethylbutanoate (7′N-5F-ADB); methyl 2-[1-(4-fluorobutyl)-1H-indazole-3-carboxamido]-3,3-dimethylbutanoate (4F-MDMB-BINACA); microinjection; morpholino oligonucleotides; synthetic cannabinoid (SC); zebrafish larvae model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Waring M.J., Arrowsmith J., Leach A.R., Leeson P.D., Mandrell S., Owen R.M., Pairaudeau G., Pennie W.D., Pickett S.D., Wang J., et al. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat. Rev. Drug Discov. 2015;14:475–486. doi: 10.1038/nrd4609. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

