Synthesis of (+) -(R)-Tiruchanduramine
- PMID: 35209136
- PMCID: PMC8880061
- DOI: 10.3390/molecules27041338
Synthesis of (+) -(R)-Tiruchanduramine
Abstract
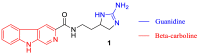
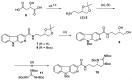
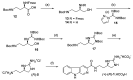
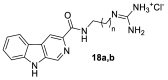
The absolute stereochemistry of the marine alkaloid (+)-(R)-tiruchanduramine was established via a convergent total synthesis in six steps and 15.5% overall yield from Fmoc-D-Dab(Boc)-OH.
Keywords: Synoicum macroglossum; guanidines; tiruchanduramine; β-carboline.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Buck M.L., Evans D.M., Evans V.A., Herkt D., Sharp H., Hollinshead J., Mitschang F., Murphy P.J., Nash R.J., Wenbourne J. A synthesis of tiruchanduramine and a reinvestigation of its glycosidase inhibitory activity. J. Tetrahedron. 2017;73:4545–4548. doi: 10.1016/j.tet.2017.06.008. - DOI
-
- Ravinder K., Reddy A.V., Krishnaiah P., Ramesh P., Ramakrishna S., Laatsch H., Venkateswarlu Y. Isolation and Synthesis of a Novel β-Carboline Guanidine Derivative Tiruchanduramine from the Indian Ascidian Synoicum macroglossum. Tetrahedron Lett. 2005;46:5475–5478. doi: 10.1016/j.tetlet.2005.06.060. - DOI
-
- Yee H.S., Fong N.T. A review of the safety and efficacy of acarbose in diabetes mellitus. Pharmacotherapy. 1996;16:792–805. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

