Ocimum basilicum L. Methanol Extract Enhances Mitochondrial Efficiency and Decreases Adipokine Levels in Maturing Adipocytes Which Regulate Macrophage Systemic Inflammation
- PMID: 35209178
- PMCID: PMC8876186
- DOI: 10.3390/molecules27041388
Ocimum basilicum L. Methanol Extract Enhances Mitochondrial Efficiency and Decreases Adipokine Levels in Maturing Adipocytes Which Regulate Macrophage Systemic Inflammation
Abstract
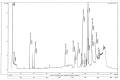
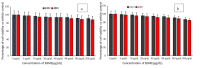
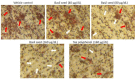
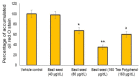
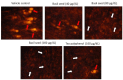
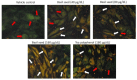
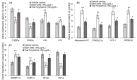
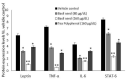
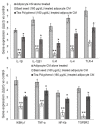
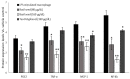
Excessive storage of lipids in visceral or ectopic sites stimulates adipokine production, which attracts macrophages. This process determines the pro- and anti-inflammatory response regulation in adipose tissue during obesity-associated systemic inflammation. The present study aimed to identify the composition of Ocimum basilicum L. (basil) seed extract and to determine its bio-efficacy on adipocyte thermogenesis or fatty acid oxidation and inhibition of lipid accumulation and adipokine secretion. Ocimum basilicum L. seed methanol extract (BSME) was utilized to analyze the cytotoxicity vs. control; lipid accumulation assay (oil red O and Nile red staining), adipogenesis and mitochondrial-thermogenesis-related gene expression vs. vehicle control were analyzed by PCR assay. In addition, vehicle control and BSME-treated adipocytes condition media were collected and treated with lipopolysaccharide (LPS)-induced macrophage to identify the macrophage polarization. The results shown that the active components present in BSME did not produce significant cytotoxicity in preadipocytes or macrophages in the MTT assay. Furthermore, oil red O and Nile red staining assay confirmed that 80 and 160 μg/dL concentrations of BSME effectively arrested lipid accumulation and inhibited adipocyte maturation, when compared with tea polyphenols. Gene expression level of adipocyte hyperplasia (CEBPα, PPARγ) and lipogenesis (LPL)-related genes have been significantly (p ≤ 0.05) downregulated, and mitochondrial-thermogenesis-associated genes (PPARγc1α, UCP-1, prdm16) have been significantly (p ≤ 0.001) upregulated. The BSME-treated, maturing, adipocyte-secreted proteins were detected with a decreased protein level of leptin, TNF-α, IL-6 and STAT-6, which are associated with insulin resistance and macrophage recruitment. The "LPS-stimulated macrophage" treated with "BSME-treated adipocytes condition media", shown with significant (p ≤ 0.001) decrease in metabolic-inflammation-related proteins-such as PGE-2, MCP-1, TNF-α and NF-κB-were majorly associated with the development of foam cell formation and progression of atherosclerotic lesion. The present findings concluded that the availability of active principles in basil seed effectively inhibit adipocyte hypertrophy, macrophage polarization, and the inflammation associated with insulin resistance and thrombosis development. Ocimum basilicum L. seed may be useful as a dietary supplement to enhance fatty acid oxidation, which aids in overcoming metabolic complications.
Keywords: adipocytes; basil seed; inflammation; lipogenesis; mitochondrial thermogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Inhibition of Lipid Accumulation and Adipokine Levels in Maturing Adipocytes by Bauhinia rufescens (Lam.) Stem Bark Extract Loaded Titanium Oxide Nanoparticles.Molecules. 2021 Nov 29;26(23):7238. doi: 10.3390/molecules26237238. Molecules. 2021. PMID: 34885819 Free PMC article.
-
Beneficial Fatty Acid Ratio of Salvia hispanica L. (Chia Seed) Potentially Inhibits Adipocyte Hypertrophy, and Decreases Adipokines Expression and Inflammation in Macrophage.Foods. 2020 Mar 22;9(3):368. doi: 10.3390/foods9030368. Foods. 2020. PMID: 32235695 Free PMC article.
-
Ononitol monohydrate enhances PRDM16 & UCP-1 expression, mitochondrial biogenesis and insulin sensitivity via STAT6 and LTB4R in maturing adipocytes.Biomed Pharmacother. 2018 Mar;99:375-383. doi: 10.1016/j.biopha.2018.01.084. Biomed Pharmacother. 2018. PMID: 29358130
-
Adipocyte-Macrophage Cross-Talk in Obesity.Adv Exp Med Biol. 2017;960:327-343. doi: 10.1007/978-3-319-48382-5_14. Adv Exp Med Biol. 2017. PMID: 28585206 Review.
-
Novel insights of dietary polyphenols and obesity.J Nutr Biochem. 2014 Jan;25(1):1-18. doi: 10.1016/j.jnutbio.2013.09.001. J Nutr Biochem. 2014. PMID: 24314860 Free PMC article. Review.
Cited by
-
Chinese medicine Jiangzhuo mixture regulates glucose and lipid metabolism in obese rats through TLR4/IκBα/NF-κB signaling pathway.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2023 Oct 7;52(5):627-635. doi: 10.3724/zdxbyxb-2023-0164. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2023. PMID: 37899401 Free PMC article. Chinese, English.
-
Supernatants from Newly Isolated Lacticaseibacillus paracasei P4 Ameliorate Adipocyte Metabolism in Differentiated 3T3-L1 Cells.Biomedicines. 2024 Dec 7;12(12):2785. doi: 10.3390/biomedicines12122785. Biomedicines. 2024. PMID: 39767692 Free PMC article.
-
Holy Basil (Ocimum sanctum L.) Flower and Fenofibrate Improve Lipid Profiles in Rats with Metabolic Dysfunction Associated Steatotic Liver Disease (MASLD): The Role of Choline Metabolism.Plants (Basel). 2024 Dec 24;14(1):13. doi: 10.3390/plants14010013. Plants (Basel). 2024. PMID: 39795274 Free PMC article.
-
6-Gingerol Ameliorates Adiposity and Inflammation in Adipose Tissue in High Fat Diet-Induced Obese Mice: Association with Regulating of Adipokines.Nutrients. 2023 Aug 4;15(15):3457. doi: 10.3390/nu15153457. Nutrients. 2023. PMID: 37571394 Free PMC article.
-
Targeting Regulation of Macrophage to Treat Metabolic Disease: Role of Phytochemicals.Cell Prolif. 2025 Jul;58(7):e70012. doi: 10.1111/cpr.70012. Epub 2025 Mar 5. Cell Prolif. 2025. PMID: 40045164 Free PMC article. Review.
References
-
- Rebollo-Hernanz M., Zhang Q., Aguilera Y., Martín-Cabrejas M.A., de Mejia E.G. Cocoa Shell Aqueous Phenolic Extract Preserves Mitochondrial Function and Insulin Sensitivity by Attenuating Inflammation between Macrophages and Adipocytes In Vitro. Mol. Nutr. Food Res. 2019;63:e1801413. doi: 10.1002/mnfr.201801413. - DOI - PubMed
-
- Meissburger B., Ukropec J., Roeder E., Beaton N., Geiger M., Teupser D., Civan B., Langhans W., Nawroth P.P., Gasperikova D., et al. Adipogenesis and insulin sensitivity in obesity are regulated by retinoid-related orphan receptor gamma. EMBO Mol. Med. 2011;3:637–651. doi: 10.1002/emmm.201100172. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

