Recovery Techniques Enabling Circular Chemistry from Wastewater
- PMID: 35209179
- PMCID: PMC8877087
- DOI: 10.3390/molecules27041389
Recovery Techniques Enabling Circular Chemistry from Wastewater
Abstract
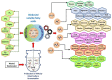
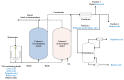
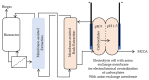
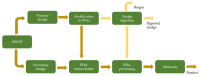
In an era where it becomes less and less accepted to just send waste to landfills and release wastewater into the environment without treatment, numerous initiatives are pursued to facilitate chemical production from waste. This includes microbial conversions of waste in digesters, and with this type of approach, a variety of chemicals can be produced. Typical for digestion systems is that the products are present only in (very) dilute amounts. For such productions to be technically and economically interesting to pursue, it is of key importance that effective product recovery strategies are being developed. In this review, we focus on the recovery of biologically produced carboxylic acids, including volatile fatty acids (VFAs), medium-chain carboxylic acids (MCCAs), long-chain dicarboxylic acids (LCDAs) being directly produced by microorganisms, and indirectly produced unsaturated short-chain acids (USCA), as well as polymers. Key recovery techniques for carboxylic acids in solution include liquid-liquid extraction, adsorption, and membrane separations. The route toward USCA is discussed, including their production by thermal treatment of intracellular polyhydroxyalkanoates (PHA) polymers and the downstream separations. Polymers included in this review are extracellular polymeric substances (EPS). Strategies for fractionation of the different fractions of EPS are discussed, aiming at the valorization of both polysaccharides and proteins. It is concluded that several separation strategies have the potential to further develop the wastewater valorization chains.
Keywords: extracellular polymeric substances; long-chain dicarboxylic acids; medium-chain carboxylic acids; separation technology; unsaturated fatty acids; volatile fatty acids.
Conflict of interest statement
There is no conflict of interest.
Figures






References
-
- Jess A., Wasserscheid P. Chemical Technology: From Principles to Products. 2nd ed. Wiley-VCH; Weinheim, Germany: 2020. p. 591.
-
- Wagemann K., Tippkötter N. Biorefineries: A Short Introduction. In: Wagemann K., Tippkötter N., editors. Biorefineries. Springer; Berlin/Heidelberg, Germany: 2019. pp. 1–11. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

