Coptisine Alleviates Imiquimod-Induced Psoriasis-like Skin Lesions and Anxiety-like Behavior in Mice
- PMID: 35209199
- PMCID: PMC8878104
- DOI: 10.3390/molecules27041412
Coptisine Alleviates Imiquimod-Induced Psoriasis-like Skin Lesions and Anxiety-like Behavior in Mice
Abstract
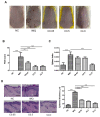
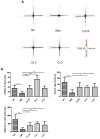
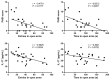
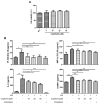
Psoriasis is a common inflammatory skin disorder, which can be associated with psychological disorders, such as anxiety and depression. This study investigated the efficacy and the mechanism of action of a natural compound coptisine using imiquimod (IMQ)-induced psoriasis mice. Coptisine reduced the severity of psoriasis-like skin lesions, decreased epidermal hyperplasia and the levels of inflammatory cytokines TNF-α, IL-17, and IL-22. Furthermore, coptisine improved IMQ-induced anxiety in mice by increasing the number of entries and time in open arms in the elevated plus maze (EPM) test. Coptisine also lowered the levels of inflammatory cytokines TNF-α and IL-1β in the prefrontal cortex of psoriasis mice. HaCaT keratinocytes and BV2 microglial cells were used to investigate the effects of coptisine in vitro. In M5-treated HaCaT cells, coptisine decreased the production of IL-6, MIP-3α/CCL20, IP-10/CXCL10, and ICAM-1 and suppressed the NF-κB signaling pathway. In LPS-stimulated BV2 cells, coptisine reduced the secretion of TNF-α and IL-1β. These findings suggest that coptisine might be a potential candidate for psoriasis treatment by improving both disease severity and psychological comorbidities.
Keywords: anxiety; coptisine; imiquimod; inflammation; psoriasis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






Similar articles
-
Daphnetin inhibits proliferation and inflammatory response in human HaCaT keratinocytes and ameliorates imiquimod-induced psoriasis-like skin lesion in mice.Biol Res. 2020 Oct 20;53(1):48. doi: 10.1186/s40659-020-00316-0. Biol Res. 2020. PMID: 33081840 Free PMC article.
-
Dang-Gui-Liu-Huang Tang a traditional herbal formula, ameliorates imiquimod-induced psoriasis-like skin inflammation in mice by inhibiting IL-22 production.Phytomedicine. 2018 Aug 1;47:48-57. doi: 10.1016/j.phymed.2018.04.051. Epub 2018 May 9. Phytomedicine. 2018. PMID: 30166108
-
Anoctamin1 Induces Hyperproliferation of HaCaT Keratinocytes and Triggers Imiquimod-Induced Psoriasis-Like Skin Injury in Mice.Int J Mol Sci. 2021 Jul 1;22(13):7145. doi: 10.3390/ijms22137145. Int J Mol Sci. 2021. PMID: 34281197 Free PMC article.
-
Datura Metel L. Ameliorates Imiquimod-Induced Psoriasis-Like Dermatitis and Inhibits Inflammatory Cytokines Production through TLR7/8-MyD88-NF-κB-NLRP3 Inflammasome Pathway.Molecules. 2019 Jun 7;24(11):2157. doi: 10.3390/molecules24112157. Molecules. 2019. PMID: 31181689 Free PMC article.
-
Proinflammatory cytokines and neuropeptides in psoriasis, depression, and anxiety.Acta Physiol (Oxf). 2025 Mar;241(3):e70019. doi: 10.1111/apha.70019. Acta Physiol (Oxf). 2025. PMID: 39960105 Review.
Cited by
-
Exploring the mechanism of Huanglian ointment in alleviating wound healing after anal fistula surgery through metabolomics and proteomics.Heliyon. 2024 Apr 16;10(9):e29809. doi: 10.1016/j.heliyon.2024.e29809. eCollection 2024 May 15. Heliyon. 2024. PMID: 38699024 Free PMC article.
-
Broussonetia papyrifera ameliorates imiquimod-induced psoriasis-like skin inflammation in mice by modulating the TLR4/NF-κB and PI3K/AKT signaling pathways.PLoS One. 2025 May 7;20(5):e0322710. doi: 10.1371/journal.pone.0322710. eCollection 2025. PLoS One. 2025. PMID: 40333872 Free PMC article.
-
Extracellular CIRP Upregulates Proinflammatory Cytokine Expression via the NF-kappaB and ERK1/2 Signaling Pathways in Psoriatic Keratinocytes.Mediators Inflamm. 2022 Sep 6;2022:5978271. doi: 10.1155/2022/5978271. eCollection 2022. Mediators Inflamm. 2022. PMID: 36110097 Free PMC article.
-
Molecular insights into the bidirectional link between anxiety and COVID-19: a combined clinical and bioinformatics approach.Front Psychiatry. 2025 Jul 22;16:1643355. doi: 10.3389/fpsyt.2025.1643355. eCollection 2025. Front Psychiatry. 2025. PMID: 40766923 Free PMC article.
-
Tanshinone I Ameliorates Psoriasis-Like Dermatitis by Suppressing Inflammation and Regulating Keratinocyte Differentiation.Drug Des Devel Ther. 2025 Jan 24;19:539-552. doi: 10.2147/DDDT.S504485. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 39876988 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

