Cysteine as a Multifaceted Player in Kidney, the Cysteine-Related Thiolome and Its Implications for Precision Medicine
- PMID: 35209204
- PMCID: PMC8874463
- DOI: 10.3390/molecules27041416
Cysteine as a Multifaceted Player in Kidney, the Cysteine-Related Thiolome and Its Implications for Precision Medicine
Abstract
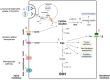
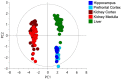
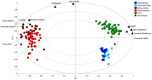
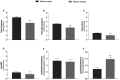
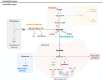
In this review encouraged by original data, we first provided in vivo evidence that the kidney, comparative to the liver or brain, is an organ particularly rich in cysteine. In the kidney, the total availability of cysteine was higher in cortex tissue than in the medulla and distributed in free reduced, free oxidized and protein-bound fractions (in descending order). Next, we provided a comprehensive integrated review on the evidence that supports the reliance on cysteine of the kidney beyond cysteine antioxidant properties, highlighting the relevance of cysteine and its renal metabolism in the control of cysteine excess in the body as a pivotal source of metabolites to kidney biomass and bioenergetics and a promoter of adaptive responses to stressors. This view might translate into novel perspectives on the mechanisms of kidney function and blood pressure regulation and on clinical implications of the cysteine-related thiolome as a tool in precision medicine.
Keywords: H2S; bioenergetics; cysteine transporters; cysteine-related thiolome; ferroptosis; glutathione; hypertension; hypoxia; kidney metabolism; lysosomes.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of the data; in the writing of the manuscript or in the decision to publish the results.
Figures
References
-
- Bowes A.D.P., Church H.N., Pennington J.A.T. Supplementary tables—amino acids. In: Allen A., editor. Bowes and Church’s Food Values of Portions Commonly Used. J.B. Lippincott; Philadelphia, CA, USA: 1994. pp. 325–377.
-
- Hirakawa D.A., Baker D.H. Sulfur amino acid nutrition of the growing puppy: Determination of dietary requirements for methionine and cystine. Nutr. Res. 1985;5:631–642. doi: 10.1016/S0271-5317(85)80244-X. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

