Carotenoids and Their Biosynthesis in Fungi
- PMID: 35209220
- PMCID: PMC8879039
- DOI: 10.3390/molecules27041431
Carotenoids and Their Biosynthesis in Fungi
Abstract
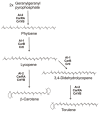
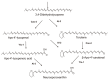
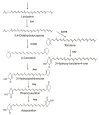
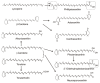
Carotenoids represent a class of pigmented terpenoids. They are distributed in all taxonomic groups of fungi. Most of the fungal carotenoids differ in their chemical structures to those from other organisms. The general function of carotenoids in heterotrophic organisms is protection as antioxidants against reactive oxygen species generated by photosensitized reactions. Furthermore, carotenoids are metabolized to apocarotenoids by oxidative cleavage. This review presents the current knowledge on fungal-specific carotenoids, their occurrence in different taxonomic groups, and their biosynthesis and conversion into trisporic acids. The outline of the different pathways was focused on the reactions and genes involved in not only the known pathways, but also suggested the possible mechanisms of reactions, which may occur in several non-characterized pathways in different fungi. Finally, efforts and strategies for genetic engineering to enhance or establish pathways for the production of various carotenoids in carotenogenic or non-carotenogenic yeasts were highlighted, addressing the most-advanced producers of each engineered yeast, which offered the highest biotechnological potentials as production systems.
Keywords: carotenogenic pathways; carotenoid biosynthesis; carotenoid distribution; carotenoid pathway engineering; reaction mechanisms; trisporic acids.
Conflict of interest statement
The author declares no conflict of interest.
Figures


References
-
- Goodwin T.W. The Biochemistry of the Carotenoids. 2nd ed. Volumn I. Chapman and Hall; London, UK: New York, NY, USA: 1980. pp. 257–290. Chapter 8.
-
- Valadon L.R.G. Carotenoids as additional taxonomic characters in fungi: A review. Trans. Br. Mycol. Soc. 1976;67:1–15. doi: 10.1016/S0007-1536(76)80001-0. - DOI
-
- Sandmann G., Misawa N. Fungal carotenoids. In: Osiewacz H.D., editor. The Mycota X. Industrial Applications. Springer; Berlin, Germany: 2002. pp. 247–262.
-
- Will O.H., III, Jankowski P., Kowacs A., Rossing W., Schneider P., Newland N.A. A comparison of photo-killing among carotene and cytochrome c accumulating strains of the smut fungus Ustilaga violacea at specific wavelengths from 400 to 600 nm. Photochem. Photobiol. 1987;45:609–615. doi: 10.1111/j.1751-1097.1987.tb07387.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

