Synthesis and Characterization of an α-Fe2O3-Decorated g-C3N4 Heterostructure for the Photocatalytic Removal of MO
- PMID: 35209230
- PMCID: PMC8877162
- DOI: 10.3390/molecules27041442
Synthesis and Characterization of an α-Fe2O3-Decorated g-C3N4 Heterostructure for the Photocatalytic Removal of MO
Abstract
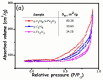
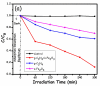
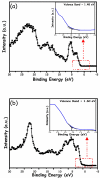
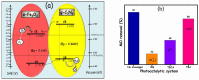
This study describes the preparation of graphitic carbon nitride (g-C3N4), hematite (α-Fe2O3), and their g-C3N4/α-Fe2O3 heterostructure for the photocatalytic removal of methyl orange (MO) under visible light illumination. The facile hydrothermal approach was utilized for the preparation of the nanomaterials. Powder X-ray diffraction (XRD), Scanning electron microscopy (SEM), Energy dispersive X-ray (EDX), and Brunauer-Emmett-Teller (BET) were carried out to study the physiochemical and optoelectronic properties of all the synthesized photocatalysts. Based on the X-ray photoelectron spectroscopy (XPS) and UV-visible diffuse reflectance (DRS) results, an energy level diagram vs. SHE was established. The acquired results indicated that the nanocomposite exhibited a type-II heterojunction and degraded the MO dye by 97%. The degradation ability of the nanocomposite was higher than that of pristine g-C3N4 (41%) and α-Fe2O3 (30%) photocatalysts under 300 min of light irradiation. The formation of a type-II heterostructure with desirable band alignment and band edge positions for efficient interfacial charge carrier separation along with a larger specific surface area was collectively responsible for the higher photocatalytic efficiency of the g-C3N4/α-Fe2O3 nanocomposite. The mechanism of the nanocomposite was also studied through results obtained from UV-vis and XPS analyses. A reactive species trapping experiment confirmed the involvement of the superoxide radical anion (O2•-) as the key reactive oxygen species for MO removal. The degradation kinetics were also monitored, and the reaction was observed to be pseudo-first order. Moreover, the sustainability of the photocatalyst was also investigated.
Keywords: MO photodegradation; alignment of energy levels; g-C3N4; g-C3N4/α-Fe2O3 nanocomposite; heterostructure (type-II).
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures










References
-
- Chang J.-S., Lin C.-Y. Decolorization kinetics of a recombinant Escherichia coli strain harboring azo-dye-decolorizing determinants from Rhodococcus sp. Biotechnol. Lett. 2001;23:631–636. doi: 10.1023/A:1010306114286. - DOI
-
- Bai Y.-N., Wang X.-N., Zhang F., Wu J., Zhang W., Lu Y.-Z., Fu L., Lau T.-C., Zeng R.J. High-rate anaerobic decolorization of methyl orange from synthetic azo dye wastewater in a methane-based hollow fiber membrane bioreactor. J. Hazard. Mater. 2020;388:121753. doi: 10.1016/j.jhazmat.2019.121753. - DOI - PubMed
-
- Kant R. Textile dyeing industry an environmental hazard. Nat. Sci. 2011;4:17027. doi: 10.4236/ns.2012.41004. - DOI
LinkOut - more resources
Full Text Sources

