Targeting of the alphav beta3 integrin complex by CAR-T cells leads to rapid regression of diffuse intrinsic pontine glioma and glioblastoma
- PMID: 35210306
- PMCID: PMC8883284
- DOI: 10.1136/jitc-2021-003816
Targeting of the alphav beta3 integrin complex by CAR-T cells leads to rapid regression of diffuse intrinsic pontine glioma and glioblastoma
Abstract
Background: Diffuse intrinsic pontine glioma (DIPG) and glioblastoma (GBM) are two highly aggressive and generally incurable gliomas with little therapeutic advancements made in the past several decades. Despite immense initial success of chimeric antigen receptor (CAR) T cells for the treatment of leukemia and lymphoma, significant headway into the application of CAR-T cells against solid tumors, including gliomas, is still forthcoming. The integrin complex alphav beta3 (αvβ3) is present on multiple and diverse solid tumor types and tumor vasculature with limited expression throughout most normal tissues, qualifying it as an appealing target for CAR-T cell-mediated immunotherapy.
Methods: Patient-derived DIPG and GBM cell lines were evaluated by flow cytometry for surface expression of αvβ3. Second-generation CAR-T cells expressing an anti-αvβ3 single-chain variable fragment were generated by retroviral transduction containing either a CD28 or 4-1BB costimulatory domain and CD3zeta. CAR-T cells were evaluated by flow cytometry for CAR expression, memory phenotype distribution, and inhibitory receptor profile. DIPG and GBM cell lines were orthotopically implanted into NSG mice via stereotactic injection and monitored with bioluminescent imaging to evaluate αvβ3 CAR-T cell-mediated antitumor responses.
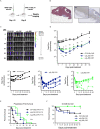
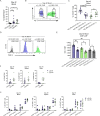
Results: We found that patient-derived DIPG cells and GBM cell lines express high levels of surface αvβ3 by flow cytometry, while αvβ3 is minimally expressed on normal tissues by RNA sequencing and protein microarray. The manufactured CAR-T cells consisted of a substantial frequency of favorable early memory cells and a low inhibitory receptor profile. αvβ3 CAR-T cells demonstrated efficient, antigen-specific tumor cell killing in both cytotoxicity assays and in in vivo models of orthotopically and stereotactically implanted DIPG and GBM tumors into relevant locations in the brain of NSG mice. Tumor responses were rapid and robust with systemic CAR-T cell proliferation and long-lived persistence associated with long-term survival. Following tumor clearance, TCF-1+αvβ3 CAR-T cells were detectable, underscoring their ability to persist and undergo self-renewal.
Conclusions: These results highlight the potential of αvβ3 CAR-T cells for immunotherapeutic treatment of aggressive brain tumors with reduced risk of on-target, off-tumor mediated toxicity due to the restricted nature of αvβ3 expression in normal tissues.
Keywords: T-lymphocytes; chimeric antigen; immunologic memory; immunotherapy; pediatrics; receptors.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DWL serves as a consultant to Juno Therapeutics/BMS, Harpoon Therapeutics, and Amgen, and his institution receives clinical trial funding from Kite Pharma/Gilead. His spouse is an employee of Karyopharm Therapeutics. DAC and DWL have a patent application pending based on the results presented in this study. All other authors declare that they have no competing interests.
Figures





Similar articles
-
Anti-tumor efficacy of anti-GD2 CAR NK-92 cells in diffuse intrinsic pontine gliomas.Front Immunol. 2023 May 12;14:1145706. doi: 10.3389/fimmu.2023.1145706. eCollection 2023. Front Immunol. 2023. PMID: 37251413 Free PMC article.
-
Intracerebroventricular B7-H3-targeting CAR T cells for diffuse intrinsic pontine glioma: a phase 1 trial.Nat Med. 2025 Mar;31(3):861-868. doi: 10.1038/s41591-024-03451-3. Epub 2025 Jan 7. Nat Med. 2025. PMID: 39775044 Free PMC article. Clinical Trial.
-
EphA3-targeted chimeric antigen receptor T cells are effective in glioma and generate curative memory T cell responses.J Immunother Cancer. 2024 Aug 7;12(8):e009486. doi: 10.1136/jitc-2024-009486. J Immunother Cancer. 2024. PMID: 39111833 Free PMC article.
-
Diffuse Intrinsic Pontine Glioma and Chimeric Antigen Receptor T-Cell Therapy: An Emerging Frontier.World Neurosurg. 2025 Feb;194:123579. doi: 10.1016/j.wneu.2024.123579. Epub 2025 Jan 22. World Neurosurg. 2025. PMID: 39694135 Review.
-
CAR T-cell therapy: a potential treatment strategy for pediatric midline gliomas.Acta Neurol Belg. 2024 Aug;124(4):1251-1261. doi: 10.1007/s13760-024-02519-8. Epub 2024 Apr 26. Acta Neurol Belg. 2024. PMID: 38669002 Review.
Cited by
-
Identifying the prognosis implication, immunotherapy response prediction value, and potential targeted compound inhibitors of integrin subunit α3 (ITGA3) in human cancers.Heliyon. 2024 Jan 6;10(2):e24236. doi: 10.1016/j.heliyon.2024.e24236. eCollection 2024 Jan 30. Heliyon. 2024. PMID: 38293430 Free PMC article.
-
CAR-T cell therapy in triple-negative breast cancer: Hunting the invisible devil.Front Immunol. 2022 Nov 22;13:1018786. doi: 10.3389/fimmu.2022.1018786. eCollection 2022. Front Immunol. 2022. PMID: 36483567 Free PMC article. Review.
-
Multifunctional Polymeric Micelles for Cancer Therapy.Polymers (Basel). 2022 Nov 10;14(22):4839. doi: 10.3390/polym14224839. Polymers (Basel). 2022. PMID: 36432965 Free PMC article. Review.
-
αvβ3 Integrin as a Link between the Development of Fibrosis and Thyroid Hormones in Systemic Sclerosis.Int J Mol Sci. 2023 May 18;24(10):8927. doi: 10.3390/ijms24108927. Int J Mol Sci. 2023. PMID: 37240272 Free PMC article.
-
Lipopolysaccharide and lipoteichoic acid regulate the PI3K/AKT pathway through osteopontin/integrin β3 to promote malignant progression of non-small cell lung cancer.J Thorac Dis. 2023 Jan 31;15(1):168-185. doi: 10.21037/jtd-22-1825. Epub 2023 Jan 16. J Thorac Dis. 2023. PMID: 36794132 Free PMC article.
References
-
- Celgene, ABECMA (idecabtagene vicleucel) [Package Insert] 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
