An immune-sympathetic neuron communication axis guides adipose tissue browning in cancer-associated cachexia
- PMID: 35210363
- PMCID: PMC8892347
- DOI: 10.1073/pnas.2112840119
An immune-sympathetic neuron communication axis guides adipose tissue browning in cancer-associated cachexia
Abstract
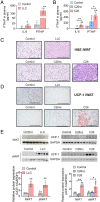
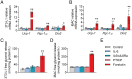
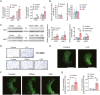
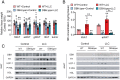
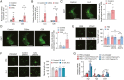
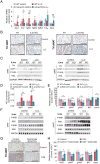
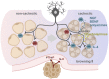
Cancer-associated cachexia (CAC) is a hypermetabolic syndrome characterized by unintended weight loss due to the atrophy of adipose tissue and skeletal muscle. A phenotypic switch from white to beige adipocytes, a phenomenon called browning, accelerates CAC by increasing the dissipation of energy as heat. Addressing the mechanisms of white adipose tissue (WAT) browning in CAC, we now show that cachexigenic tumors activate type 2 immunity in cachectic WAT, generating a neuroprotective environment that increases peripheral sympathetic activity. Increased sympathetic activation, in turn, results in increased neuronal catecholamine synthesis and secretion, β-adrenergic activation of adipocytes, and induction of WAT browning. Two genetic mouse models validated this progression of events. 1) Interleukin-4 receptor deficiency impeded the alternative activation of macrophages, reduced sympathetic activity, and restrained WAT browning, and 2) reduced catecholamine synthesis in peripheral dopamine β-hydroxylase (DBH)-deficient mice prevented cancer-induced WAT browning and adipose atrophy. Targeting the intraadipose macrophage-sympathetic neuron cross-talk represents a promising therapeutic approach to ameliorate cachexia in cancer patients.
Keywords: adipose tissue; browning; cancer cachexia; immunometabolism; macrophage.
Copyright © 2022 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures
References
-
- Petruzzelli M., et al. , A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 20, 433–447 (2014). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

