HTLV-1 activates YAP via NF-κB/p65 to promote oncogenesis
- PMID: 35210364
- PMCID: PMC8892356
- DOI: 10.1073/pnas.2115316119
HTLV-1 activates YAP via NF-κB/p65 to promote oncogenesis
Abstract
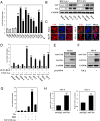
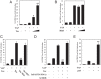
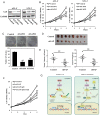
Adult T-cell leukemia/lymphoma (ATL) is an aggressive malignancy caused by human T-cell leukemia virus type 1 (HTLV-1) infection. HTLV-1 exerts its oncogenic functions by interacting with signaling pathways involved in cell proliferation and transformation. Dysregulation of the Hippo/YAP pathway is associated with multiple cancers, including virus-induced malignancies. In the present study, we observe that expression of YAP, which is the key effector of Hippo signaling, is elevated in ATL cells by the action of the HTLV-1 Tax protein. YAP transcriptional activity is remarkably enhanced in HTLV-1-infected cells and ATL patients. In addition, Tax activates the YAP protein via a mechanism involving the NF-κB/p65 pathway. As a mechanism for this cross talk between the Hippo and NF-κB pathways, we found that p65 abrogates the interaction between YAP and LATS1, leading to suppression of YAP phosphorylation, inhibition of ubiquitination-dependent degradation of YAP, and YAP nuclear accumulation. Finally, knockdown of YAP suppresses the proliferation of ATL cells in vitro and tumor formation in ATL-engrafted mice. Taken together, our results suggest that p65-induced YAP activation is essential for ATL pathogenesis and implicate YAP as a potential therapeutic target for ATL treatment.
Keywords: ATL; HTLV-1; Tax; YAP; p65.
Copyright © 2022 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Matsuoka M., Jeang K. T., Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat. Rev. Cancer 7, 270–280 (2007). - PubMed
-
- Hall W. W., Fujii M., Deregulation of cell-signaling pathways in HTLV-1 infection. Oncogene 24, 5965–5975 (2005). - PubMed
-
- Nicot C., Harrod R. L., Ciminale V., Franchini G., Human T-cell leukemia/lymphoma virus type 1 nonstructural genes and their functions. Oncogene 24, 6026–6034 (2005). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

