Chip-based multimodal super-resolution microscopy for histological investigations of cryopreserved tissue sections
- PMID: 35210400
- PMCID: PMC8873254
- DOI: 10.1038/s41377-022-00731-w
Chip-based multimodal super-resolution microscopy for histological investigations of cryopreserved tissue sections
Abstract
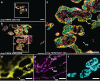
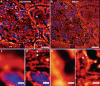
Histology involves the observation of structural features in tissues using a microscope. While diffraction-limited optical microscopes are commonly used in histological investigations, their resolving capabilities are insufficient to visualize details at subcellular level. Although a novel set of super-resolution optical microscopy techniques can fulfill the resolution demands in such cases, the system complexity, high operating cost, lack of multi-modality, and low-throughput imaging of these methods limit their wide adoption for histological analysis. In this study, we introduce the photonic chip as a feasible high-throughput microscopy platform for super-resolution imaging of histological samples. Using cryopreserved ultrathin tissue sections of human placenta, mouse kidney, pig heart, and zebrafish eye retina prepared by the Tokuyasu method, we demonstrate diverse imaging capabilities of the photonic chip including total internal reflection fluorescence microscopy, intensity fluctuation-based optical nanoscopy, single-molecule localization microscopy, and correlative light-electron microscopy. Our results validate the photonic chip as a feasible imaging platform for tissue sections and pave the way for the adoption of super-resolution high-throughput multimodal analysis of cryopreserved tissue samples both in research and clinical settings.
© 2022. The Author(s).
Conflict of interest statement
BSA has applied for a patent for chip-based optical nanoscopy and he is co-founder of the company Chip NanoImaging AS, which commercializes on-chip super-resolution microscopy systems.
Figures



References
-
- Redman CWG, et al. Does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta. 2012;33:S48–S54. - PubMed
-
- Lange K. Fundamental role of microvilli in the main functions of differentiated cells: outline of an universal regulating and signaling system at the cell periphery. J. Cell. Physiol. 2011;226:896–927. - PubMed
-
- Unwin PNT, Zampighi G. Structure of the junction between communicating cells. Nature. 1980;283:545–549. - PubMed
-
- Inaga S, et al. Rapid three-dimensional analysis of renal biopsy sections by low vacuum scanning electron microscopy. Arch. Histol. Cytol. 2011;73:113–125. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

