Latexin deficiency attenuates adipocyte differentiation and protects mice against obesity and metabolic disorders induced by high-fat diet
- PMID: 35210404
- PMCID: PMC8873487
- DOI: 10.1038/s41419-022-04636-9
Latexin deficiency attenuates adipocyte differentiation and protects mice against obesity and metabolic disorders induced by high-fat diet
Abstract
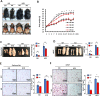
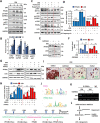
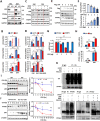
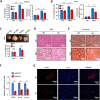
Obesity is a risk factor for many chronic diseases, and is associated with increased incidence rate of type 2 diabetes, hypertension, dyslipidemia and cardiovascular diseases. Adipocyte differentiation play critical role during development of obesity. Latexin (LXN), a mammalian carboxypeptidase inhibitor, plays important role in the proliferation and differentiation of stem cells, and highlights as a differentiation-associated gene that was significantly downregulated in prostate stem cells and whose expression increases through differentiation. However, it is unclear whether LXN is involved in adipocyte differentiation. The aim of this study was to evaluate the role of LXN on adipocyte differentiation, as well as its effects on high fat-induced obesity and metabolic disorders. In this study, we determine the expression of LXN in adipose tissue of lean and fat mice by Western blot, qPCR and immunohistochemistry. We found that LXN in fat tissues was continuous increased during the development of diet-induced obesity. We fed wild-type (WT) and LXN-/-mice with high-fat diet (HFD) to study the effects of LXN on obesity and related metabolic functions. We found that mice deficient in LXN showed resistance against high-fat diet (HFD)-induced obesity, glucose tolerance, insulin tolerance and hepatic steatosis. In vitro studies indicated that LXN was highly induced during adipocyte differentiation, and positively regulated adipocyte differentiation and adipogenesis in 3T3-L1 cells and primary preadipocytes. Functional analysis revealed that the expression of LXN was positively regulated by mTOR/RXR/PPARɤ signaling pathway during the differentiation of adipocytes, while LXN deletion decreased the protein level of PPARɤ in adipocyte through enhancing FABP4 mediated ubiquitination, which led to impaired adipocyte differentiation and lipogenesis. Collectively, our data provide evidence that LXN is a key positive regulator of adipocyte differentiation, and therapeutics targeting LXN could be effective in preventing obesity and its associated disorders in clinical settings.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376:254–66. - PubMed
-
- Piche ME, Tchernof A, Despres JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circulation Res. 2020;126:1477–1500. - PubMed
-
- Cinti S. The adipose organ. Prostaglandins, leukotrienes, Essent Fat acids. 2005;73:9–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 31660242/National Natural Science Foundation of China (National Science Foundation of China)
- 81770310/National Natural Science Foundation of China (National Science Foundation of China)
- 82104496/National Natural Science Foundation of China (National Science Foundation of China)
- 2017GXNSFFA198003/Natural Science Foundation of Guangxi Zhuang Autonomous Region
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

