c-Myb protects cochlear hair cells from cisplatin-induced damage via the PI3K/Akt signaling pathway
- PMID: 35210433
- PMCID: PMC8873213
- DOI: 10.1038/s41420-022-00879-9
c-Myb protects cochlear hair cells from cisplatin-induced damage via the PI3K/Akt signaling pathway
Abstract
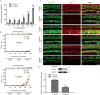
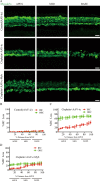
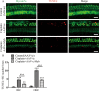
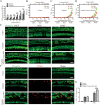
The transcription factor c-Myb is vital for cell survival, proliferation, differentiation, and apoptosis. We have previously reported that c-Myb knockdown exacerbates neomycin-induced damage to cochlea cells. However, the function and regulation of c-Myb in the mammalian inner ear remains unclear. Here, we first found that the expression of c-Myb in cochlear HCs was downregulated after cisplatin damage in vivo. Next, to investigate the role of c-Myb in HCs treated with cisplatin, the recombinant virus AAV-ie-CAG-Myb-HA (AAV-c-Myb) that overexpresses c-Myb was constructed and transfected into HCs. The protein expression of c-Myb was effectively up-regulated in cultured cochlear HCs after the virus transfection, which increased cochlear HC viability, decreased HC apoptosis and reduced intracellular reactive oxygen species (ROS) levels after cisplatin injury in vitro. The overexpression of c-Myb in HCs after AAV-c-Myb transfection in vivo also promoted HC survival, improved the hearing function of mice and reduced HC apoptosis after cisplatin injury. Furthermore, c-Myb-HC conditional knockout mice (Prestin; c-Myb-cKO) in which c-Myb expression is downregulated only in cochlear OHCs were generated and the cisplatin-induced HCs loss, apoptosis and hearing deficit were all exacerbated in Prestin; c-Myb-cKO mice treated with cisplatin in vivo. Finally, mechanistic studies showed that upregulation of the PI3K/Akt signaling pathway by c-Myb contributed to the increased HC survival after cisplatin exposure in vitro. The findings from this work suggest that c-Myb might serve as a new target for the prevention of cisplatin-induced HC damage and hearing loss.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
c-Myb knockdown increases the neomycin-induced damage to hair-cell-like HEI-OC1 cells in vitro.Sci Rep. 2017 Jan 23;7:41094. doi: 10.1038/srep41094. Sci Rep. 2017. PMID: 28112219 Free PMC article.
-
Wnt activation protects against neomycin-induced hair cell damage in the mouse cochlea.Cell Death Dis. 2016 Mar 10;7(3):e2136. doi: 10.1038/cddis.2016.35. Cell Death Dis. 2016. PMID: 26962686 Free PMC article.
-
Hippo/YAP signaling pathway protects against neomycin-induced hair cell damage in the mouse cochlea.Cell Mol Life Sci. 2022 Jan 19;79(2):79. doi: 10.1007/s00018-021-04029-9. Cell Mol Life Sci. 2022. PMID: 35044530 Free PMC article.
-
Cochlear hair cell regeneration after noise-induced hearing loss: Does regeneration follow development?Hear Res. 2017 Jun;349:182-196. doi: 10.1016/j.heares.2016.12.011. Epub 2016 Dec 26. Hear Res. 2017. PMID: 28034617 Free PMC article. Review.
-
Hair cell regeneration from inner ear progenitors in the mammalian cochlea.Am J Stem Cells. 2020 Jun 15;9(3):25-35. eCollection 2020. Am J Stem Cells. 2020. PMID: 32699655 Free PMC article. Review.
Cited by
-
Potential application value of pigment epithelium-derived factor in sensorineural hearing loss.Front Neurosci. 2023 Dec 14;17:1302124. doi: 10.3389/fnins.2023.1302124. eCollection 2023. Front Neurosci. 2023. PMID: 38164244 Free PMC article. Review.
-
Pathological mechanisms of connexin26-related hearing loss: Potassium recycling, ATP-calcium signaling, or energy supply?Front Mol Neurosci. 2022 Sep 15;15:976388. doi: 10.3389/fnmol.2022.976388. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36187349 Free PMC article. Review.
-
Kölliker's organ-supporting cells and cochlear auditory development.Front Mol Neurosci. 2022 Oct 11;15:1031989. doi: 10.3389/fnmol.2022.1031989. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36304996 Free PMC article. Review.
-
Targeted Next-Generation Sequencing Identified Novel Compound Heterozygous Variants in the PTPRQ Gene Causing Autosomal Recessive Hearing Loss in a Chinese Family.Front Genet. 2022 Jul 8;13:884522. doi: 10.3389/fgene.2022.884522. eCollection 2022. Front Genet. 2022. PMID: 35899188 Free PMC article.
-
ATOX1 Promotes Hepatocellular Carcinoma Carcinogenesis via Activation of the c-Myb/PI3K/AKT Signaling Pathway.J Clin Transl Hepatol. 2025 Aug 28;13(8):630-643. doi: 10.14218/JCTH.2024.00422. Epub 2025 Jul 7. J Clin Transl Hepatol. 2025. PMID: 40862287 Free PMC article.
References
-
- Tanna RJ, Lin JW, De Jesus O. Sensorineural Hearing Loss. StatPearls. Treasure Island (FL) 2021. - PubMed
-
- Barabas K, Milner R, Lurie D, Adin C. Cisplatin: a review of toxicities and therapeutic applications. Vet. Comp. Oncol. 2008;6:1–18. - PubMed
-
- van Ruijven MW, de Groot JC, Klis SF, Smoorenburg GF. The cochlear targets of cisplatin: an electrophysiological and morphological time-sequence study. Hearing Res. 2005;205:241–8. - PubMed
Grants and funding
- 201909189/Taishan Scholar Foundation of Shandong Province
- ts20130913/Taishan Scholar Foundation of Shandong Province
- ZR2020ZD39/Natural Science Foundation of Shandong Province (Shandong Provincial Natural Science Foundation)
- ZR2021ZD40/Natural Science Foundation of Shandong Province (Shandong Provincial Natural Science Foundation)
- 82030029,81970882/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources

