Improving the efficiency and effectiveness of an industrial SARS-CoV-2 diagnostic facility
- PMID: 35210470
- PMCID: PMC8873195
- DOI: 10.1038/s41598-022-06873-6
Improving the efficiency and effectiveness of an industrial SARS-CoV-2 diagnostic facility
Abstract
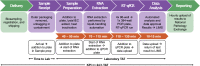
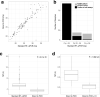
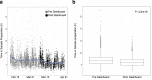
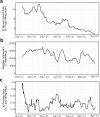
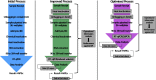
On 11th March 2020, the UK government announced plans for the scaling of COVID-19 testing, and on 27th March 2020 it was announced that a new alliance of private sector and academic collaborative laboratories were being created to generate the testing capacity required. The Cambridge COVID-19 Testing Centre (CCTC) was established during April 2020 through collaboration between AstraZeneca, GlaxoSmithKline, and the University of Cambridge, with Charles River Laboratories joining the collaboration at the end of July 2020. The CCTC lab operation focussed on the optimised use of automation, introduction of novel technologies and process modelling to enable a testing capacity of 22,000 tests per day. Here we describe the optimisation of the laboratory process through the continued exploitation of internal performance metrics, while introducing new technologies including the Heat Inactivation of clinical samples upon receipt into the laboratory and a Direct to PCR protocol that removed the requirement for the RNA extraction step. We anticipate that these methods will have value in driving continued efficiency and effectiveness within all large scale viral diagnostic testing laboratories.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- WHO. WHO Director-General's opening remarks at the media briefing on COVID-19—11 March 2020, https://www.who.int/director-general/speeches/detail/who-director-genera... (2020).
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

