Ampicillin dosing in premature infants for early-onset sepsis: exposure-driven efficacy, safety, and stewardship
- PMID: 35210541
- PMCID: PMC9262754
- DOI: 10.1038/s41372-022-01344-2
Ampicillin dosing in premature infants for early-onset sepsis: exposure-driven efficacy, safety, and stewardship
Abstract
Objective: Define optimal ampicillin dosing for empiric early-onset sepsis (EOS) therapy in preterm neonates.
Study design: We simulated ampicillin concentrations in newborns (birthweight < 1500 g; gestational age 22-27 weeks), summarizing three 48 h regimens: high 100 mg/kg Q8hr, medium 100 mg/kg Q12hr, and standard 50 mg/kg Q12hr. Concentration data were analyzed for concentration above minimum inhibitory concentration (MIC), below neurotoxicity threshold (Cmax ≤ 140 mcg/mL), and duration limited to 48 h.
Results: Among 34,689 newborns, all dosing regimens provided concentrations above MIC through 48 h, but Cmax exceeded the neurotoxicity threshold. With the 4-dose standard regimen, >90% maintained concentrations >MIC beyond 48 h. With the 2-dose regimen, newborns maintained the mean concentration >MIC within the 48 h culture window and below neurotoxicity level. Infants 22-24 weeks' gestation had higher drug concentrations and more prolonged exposure duration than 25-27 weeks' gestation.
Conclusions: For EOS in preterm infants, two ampicillin doses (50 mg/kg) provided optimal bactericidal exposures, while minimizing potential toxicity.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Figures
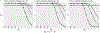
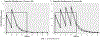
References
-
- Schrag SJ, Farley MM, Petit S, Reingold A, Weston EJ, Pondo T, et al. Epidemiology of invasive early-onset neonatal sepsis, 2005 to 2014. Pediatrics. 2016;138:e20162013. - PubMed

