Predicting plant growth response under fluctuating temperature by carbon balance modelling
- PMID: 35210545
- PMCID: PMC8873469
- DOI: 10.1038/s42003-022-03100-w
Predicting plant growth response under fluctuating temperature by carbon balance modelling
Abstract
Quantification of system dynamics is a central aim of mathematical modelling in biology. Defining experimentally supported functional relationships between molecular entities by mathematical terms enables the application of computational routines to simulate and analyse the underlying molecular system. In many fields of natural sciences and engineering, trigonometric functions are applied to describe oscillatory processes. As biochemical oscillations occur in many aspects of biochemistry and biophysics, Fourier analysis of metabolic functions promises to quantify, describe and analyse metabolism and its reaction towards environmental fluctuations. Here, Fourier polynomials were developed from experimental time-series data and combined with block diagram simulation of plant metabolism to study heat shock response of photosynthetic CO2 assimilation and carbohydrate metabolism in Arabidopsis thaliana. Simulations predicted a stabilising effect of reduced sucrose biosynthesis capacity and increased capacity of starch biosynthesis on carbon assimilation under transient heat stress. Model predictions were experimentally validated by quantifying plant growth under such stress conditions. In conclusion, this suggests that Fourier polynomials represent a predictive mathematical approach to study dynamic plant-environment interactions.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
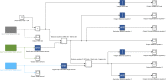
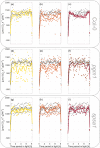

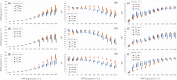

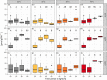



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

