In-depth comparison of the metabolic and pharmacokinetic behaviour of the structurally related synthetic cannabinoids AMB-FUBINACA and AMB-CHMICA in rats
- PMID: 35210552
- PMCID: PMC8873228
- DOI: 10.1038/s42003-022-03113-5
In-depth comparison of the metabolic and pharmacokinetic behaviour of the structurally related synthetic cannabinoids AMB-FUBINACA and AMB-CHMICA in rats
Abstract
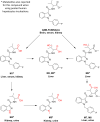
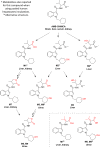
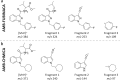
Synthetic cannabinoids receptor agonists (SCRAs) are often almost completely metabolised, and hence their pharmacokinetics should be carefully evaluated for determining the most adequate biomarker in toxicological analysis. Two structurally related SCRAs, AMB-FUBINACA and AMB-CHMICA, were selected to evaluate their in vivo metabolism and pharmacokinetics using male Sprague-Dawley rats. Brain, liver, kidney, blood (serum) and urine samples were collected at different times to assess the differences in metabolism, metabolic reactions, tissue distribution and excretion. Both compounds experimented O-demethyl reaction, which occurred more rapidly for AMB-FUBINACA. The parent compounds and O-demethyl metabolites were highly bioaccumulated in liver, and were still detected in this tissue 48 h after injection. The different indazole/indole N-functionalisation produced diverse metabolic reactions in this moiety and thus, different urinary metabolites were formed. Out of the two compounds, AMB-FUBINACA seemed to easily cross the blood-brain barrier, presenting higher brain/serum concentrations ratio than AMB-CHMICA.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
A Systematic Study of the In Vitro Pharmacokinetics and Estimated Human In Vivo Clearance of Indole and Indazole-3-Carboxamide Synthetic Cannabinoid Receptor Agonists Detected on the Illicit Drug Market.Molecules. 2021 Mar 5;26(5):1396. doi: 10.3390/molecules26051396. Molecules. 2021. PMID: 33807614 Free PMC article.
-
Pharmacology of Valinate and tert-Leucinate Synthetic Cannabinoids 5F-AMBICA, 5F-AMB, 5F-ADB, AMB-FUBINACA, MDMB-FUBINACA, MDMB-CHMICA, and Their Analogues.ACS Chem Neurosci. 2016 Sep 21;7(9):1241-54. doi: 10.1021/acschemneuro.6b00137. Epub 2016 Jul 27. ACS Chem Neurosci. 2016. PMID: 27421060
-
Fatal intoxication with new synthetic cannabinoids AMB-FUBINACA and EMB-FUBINACA.Clin Toxicol (Phila). 2019 Nov;57(11):1103-1108. doi: 10.1080/15563650.2019.1580371. Epub 2019 Feb 26. Clin Toxicol (Phila). 2019. PMID: 30806094
-
Overview of Synthetic Cannabinoids ADB-FUBINACA and AMB-FUBINACA: Clinical, Analytical, and Forensic Implications.Pharmaceuticals (Basel). 2021 Feb 25;14(3):186. doi: 10.3390/ph14030186. Pharmaceuticals (Basel). 2021. PMID: 33669071 Free PMC article. Review.
-
Lethal case of myocardial ischemia following overdose of the synthetic cannabinoid ADB-FUBINACA.Leg Med (Tokyo). 2022 Feb;54:102004. doi: 10.1016/j.legalmed.2021.102004. Epub 2021 Dec 20. Leg Med (Tokyo). 2022. PMID: 34952451 Review.
Cited by
-
Highly-sensitive detection of CP-type synthetic cannabinoids from e-cigarettes by a novel Zn/Bi bimetallic organic framework-derived ZnO-Bi2O3 heterojunctions sensing platform.Mikrochim Acta. 2024 Nov 20;191(12):750. doi: 10.1007/s00604-024-06832-0. Mikrochim Acta. 2024. PMID: 39565474
-
Off-target pharmacological profiling of synthetic cannabinoid receptor agonists including AMB-FUBINACA, CUMYL-PINACA, PB-22, and XLR-11.Front Psychiatry. 2022 Dec 15;13:1048836. doi: 10.3389/fpsyt.2022.1048836. eCollection 2022. Front Psychiatry. 2022. PMID: 36590635 Free PMC article.
-
Advances on Bioanalysis: Recent Approaches in the Determination of Biomarkers, Drugs of Abuse and Medicines.Molecules. 2022 May 17;27(10):3188. doi: 10.3390/molecules27103188. Molecules. 2022. PMID: 35630663 Free PMC article.
References
-
- European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2021: Trends and Developments (EMCDDA, 2021).
-
- Krotulski AJ, Mohr ALA, Diamond FX, Logan BK. Detection and characterization of the new synthetic cannabinoid APP‐BINACA in forensic casework. Drug Test. Anal. 2020;12:136–144. - PubMed
-
- Halter S, et al. Cumyl‐CBMICA: A new synthetic cannabinoid receptor agonist containing a cyclobutyl methyl side chain. Drug Test. Anal. 2021;13:208–216. - PubMed
-
- Cannaert A, Storme J, Franz F, Auwärter V, Stove CP. Detection and activity profiling of synthetic cannabinoids and their metabolites with a newly developed bioassay. Anal. Chem. 2016;88:11476–11485. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

