The initial rate of tumour response to vismodegib treatment, can predict a complete response outcome for periocular LA-BCC
- PMID: 35210571
- PMCID: PMC9905569
- DOI: 10.1038/s41433-022-01982-y
The initial rate of tumour response to vismodegib treatment, can predict a complete response outcome for periocular LA-BCC
Abstract
Purpose: To establish a model to predict treatment outcome of periocular locally advanced basal cell carcinoma (POLA BCC) based on initial response to treatment with vismodegib (ErivedgeTM), a sonic hedgehog inhibitor.
Design: Subgroup analysis of data from the STEVIE study database.
Methods: Analysis of medical history, treatment protocol, and treatment outcome of POLA BCC tumours in a STEVIE study population of 244 POLA BCC patients treated with ≥1 dose of vismodegib.
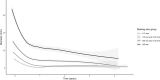
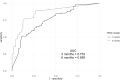
Results: A predictive model for complete response (CR) was established based on the initial treatment response. A cutoff value of 20% reduction in tumour size at 3 months of treatment identified the patients with a high probability (82.76%) to achieve CR. A second cutoff value of 67.7% reduction in tumour size at 6 months of treatment improved the prediction to a 95.42% probability of a CR outcome.
Conclusions: A treatment model was constructed based on the prediction of a CR outcome and the initial response to vismodegib treatment at 3 and 6 months. The study result provide significant new insights can facilitate decision-making on treatment management according to tumour response in patients with POLA BCC.
© 2022. The Author(s), under exclusive licence to The Royal College of Ophthalmologists.
Conflict of interest statement
Dr. Fenig reported serving as an investigator in the Safety Events in Vismodegib (STEVIE) study. No other disclosures were reported.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

