Association of Drug-Metabolizing Enzyme and Transporter Gene Polymorphisms and Lipid-Lowering Response to Statins in Thai Patients with Dyslipidemia
- PMID: 35210819
- PMCID: PMC8860396
- DOI: 10.2147/PGPM.S346093
Association of Drug-Metabolizing Enzyme and Transporter Gene Polymorphisms and Lipid-Lowering Response to Statins in Thai Patients with Dyslipidemia
Abstract
Purpose: Statins are increasingly widely used in the primary and secondary prevention of cardiovascular disease. However, there is an inter-individual variation in statin response among patients. The study aims to determine the association between genetic variations in drug-metabolizing enzyme and transporter (DMET) genes and lipid-lowering response to a statin in Thai patients with hyperlipidemia.
Patients and methods: Seventy-nine patients who received statin at steady-state concentrations were recruited. Serum lipid profile was measured at baseline and repeated after 4-month on a statin regimen. The genotype profile of 1936 DMET markers was obtained using Affymetrix DMET Plus genotyping microarrays.
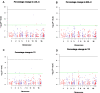
Results: In this DMET microarray platform, five variants; SLCO1B3 (rs4149117, rs7311358, and rs2053098), QPRT (rs13331798), and SLC10A2 (rs188096) showed a suggestive association with LDL-cholesterol-lowering response. HDL-cholesterol-lowering responses were found to be related to CYP7A1 gene variant (rs12542233). Seven variants, SLCO1B3 (rs4149117, rs7311358, and rs2053098); SULT1E1 (rs3736599 and rs3822172); and ABCB11 (rs4148768 and rs3770603), were associated with the total cholesterol-lowering response. One variant of the ABCB4 gene (rs2109505) was significantly associated with triglyceride-lowering response.
Conclusion: This pharmacogenomic study identifies new genetic variants of DMET genes that are associated with the lipid-lowering response to statins. Genetic polymorphisms in DMET genes may impact the pharmacokinetics and lipid-lowering response to statin. The validation studies confirmations are needed in future pharmacogenomic studies.
Keywords: drug transporters; drug-metabolizing enzymes; gene polymorphisms; statin.
© 2022 Vanwong et al.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
Similar articles
-
Pharmacogenomic Study Reveals New Variants of Drug Metabolizing Enzyme and Transporter Genes Associated with Steady-State Plasma Concentrations of Risperidone and 9-Hydroxyrisperidone in Thai Autism Spectrum Disorder Patients.Front Pharmacol. 2016 Dec 2;7:475. doi: 10.3389/fphar.2016.00475. eCollection 2016. Front Pharmacol. 2016. PMID: 28018217 Free PMC article.
-
Association of genetic variations with pharmacokinetics and lipid-lowering response to atorvastatin in healthy Korean subjects.Drug Des Devel Ther. 2017 Apr 4;11:1135-1146. doi: 10.2147/DDDT.S131487. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28435225 Free PMC article.
-
Late response to rosuvastatin and statin-related myalgia due to SLCO1B1, SLCO1B3, ABCB11, and CYP3A5 variants in a patient with Familial Hypercholesterolemia: a case report.Ann Transl Med. 2021 Jan;9(1):76. doi: 10.21037/atm-20-5540. Ann Transl Med. 2021. PMID: 33553369 Free PMC article.
-
Pharmacogenomics of statins: lipid response and other outcomes in Brazilian cohorts.Pharmacol Rep. 2022 Feb;74(1):47-66. doi: 10.1007/s43440-021-00319-y. Epub 2021 Aug 17. Pharmacol Rep. 2022. PMID: 34403130 Review.
-
2017 Taiwan lipid guidelines for high risk patients.J Formos Med Assoc. 2017 Apr;116(4):217-248. doi: 10.1016/j.jfma.2016.11.013. Epub 2017 Feb 24. J Formos Med Assoc. 2017. PMID: 28242176 Review.
Cited by
-
Determination of the latent geometry of atorvastatin pharmacokinetics by transfer entropy to identify bottlenecks.BMC Pharmacol Toxicol. 2025 Jun 25;26(Suppl 1):123. doi: 10.1186/s40360-025-00948-6. BMC Pharmacol Toxicol. 2025. PMID: 40563110 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

