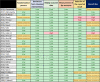
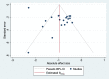
Study Design Characteristics and Endpoints for Enriched Enrollment Randomized Withdrawal Trials for Chronic Pain Patients: A Systematic Review
- PMID: 35210848
- PMCID: PMC8860756
- DOI: 10.2147/JPR.S334840
Study Design Characteristics and Endpoints for Enriched Enrollment Randomized Withdrawal Trials for Chronic Pain Patients: A Systematic Review
Abstract
Enriched enrollment randomized withdrawal (EERW) pain trials are designed to include only responders with considerable pain relief without unacceptable side effects into the randomized phase. There are no recommendations for primary endpoints in such trials. Our objective was to propose recommendations based on assessment of trial characteristics, endpoints and effect sizes in EERW pain trials. We conducted a systematic review by searching electronic databases up to June 2020 for EERW trials comparing an analgesic with a placebo in adults suffering from chronic pain. A total of 28 trials met our criteria, involving 13662 patients in the open or single-blind phase and 7937 patients in the double-blind phase. As primary endpoint 18 trials used pain intensity measured with the visual analogue scale (VAS) or the 11-point numerical rating scale (NRS); 1 trial used a 4-point NRS. Loss of therapeutic response (LTR) was used in 1 trial and time to LTR was used in 8 trials as primary endpoint. Definitions of time to LTR differed considerably between trials. Only 2 out of 8 trials using time to LTR as primary endpoint reported the percentage of patients experiencing a minimum pain relief of 50%, compared to 14 out of 18 trials using NRS or VAS. Due to the complexity and diversity of time to LTR in EERW pain trials, we propose to use the NRS as primary endpoint with conservative imputation methods, and to use time to LTR as secondary endpoint.
Keywords: EERW trials; analgesics; endpoints; pain; systematic review.
© 2022 Kopsky et al.
Conflict of interest statement
David J Kopsky reports pending patents “Topical phenytoin for use in the treatment of peripheral neuropathic pain (WO2018106107)” and “Topical pharmaceutical composition containing phenytoin and a (co-)analgesic for the treatment of chronic pain (WO2018106108)”. This systematic review is outside the scope of these patent applications. Maybe in the future a topical might be evaluated by an EERW trial. The authors have no other conflicts of interest related to this work.
Figures
Similar articles
-
A comparison between enriched and nonenriched enrollment randomized withdrawal trials of opioids for chronic noncancer pain.Pain Res Manag. 2011 Sep-Oct;16(5):337-51. doi: 10.1155/2011/465281. Pain Res Manag. 2011. PMID: 22059206 Free PMC article. Review.
-
Impact of responder definition on the enriched enrollment randomized withdrawal trial design for establishing proof of concept in neuropathic pain.Pain. 2011 Mar;152(3):514-521. doi: 10.1016/j.pain.2010.10.050. Epub 2010 Dec 23. Pain. 2011. PMID: 21185118 Clinical Trial.
-
A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis.J Neurol. 2013 Apr;260(4):984-97. doi: 10.1007/s00415-012-6739-4. Epub 2012 Nov 21. J Neurol. 2013. PMID: 23180178 Clinical Trial.
-
Once daily controlled-release pregabalin in the treatment of patients with fibromyalgia: a phase III, double-blind, randomized withdrawal, placebo-controlled study.Curr Med Res Opin. 2014 Oct;30(10):2069-83. doi: 10.1185/03007995.2014.928275. Epub 2014 Jun 27. Curr Med Res Opin. 2014. PMID: 24867298 Clinical Trial.
-
Review of Acute Treatment of Migraine Trial Results With the New FDA Endpoints: Design Implications for Future Trials.Headache. 2019 May;59(5):819-824. doi: 10.1111/head.13511. Epub 2019 Apr 6. Headache. 2019. PMID: 30953576 Free PMC article. Review.
Cited by
-
Efficacy and safety of vixotrigine in idiopathic or diabetes-associated painful small fibre neuropathy (CONVEY): a phase 2 placebo-controlled enriched-enrolment randomised withdrawal study.EClinicalMedicine. 2023 Apr 27;59:101971. doi: 10.1016/j.eclinm.2023.101971. eCollection 2023 May. EClinicalMedicine. 2023. PMID: 37152360 Free PMC article.
-
Clinical and electromyographic signals analysis about the effect of space-adjustment splint on overerupted maxillary molars.BMC Oral Health. 2024 Mar 2;24(1):296. doi: 10.1186/s12903-024-04039-6. BMC Oral Health. 2024. PMID: 38431564 Free PMC article.
-
Physical exercise improved muscle strength and pain on neck and shoulder in military pilots.Front Physiol. 2022 Sep 2;13:973304. doi: 10.3389/fphys.2022.973304. eCollection 2022. Front Physiol. 2022. PMID: 36117716 Free PMC article.
References
-
- Campbell J, King NB. ‘Unsettling circularity’: clinical trial enrichment and the evidentiary politics of chronic pain. BioSocieties. 2017;12:191–216. doi:10.1057/biosoc.2016.7 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous