Imaging of Reactive Astrogliosis by Positron Emission Tomography
- PMID: 35210989
- PMCID: PMC8862631
- DOI: 10.3389/fnins.2022.807435
Imaging of Reactive Astrogliosis by Positron Emission Tomography
Abstract
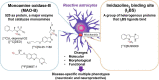
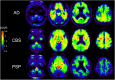
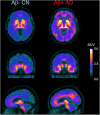
Many neurodegenerative diseases are neuropathologically characterized by neuronal loss, gliosis, and the deposition of misfolded proteins such as β-amyloid (Aβ) plaques and tau tangles in Alzheimer's disease (AD). In postmortem AD brains, reactive astrocytes and activated microglia are observed surrounding Aβ plaques and tau tangles. These activated glial cells secrete pro-inflammatory cytokines and reactive oxygen species, which may contribute to neurodegeneration. Therefore, in vivo imaging of glial response by positron emission tomography (PET) combined with Aβ and tau PET would provide new insights to better understand the disease process, as well as aid in the differential diagnosis, and monitoring glial response disease-specific therapeutics. There are two promising targets proposed for imaging reactive astrogliosis: monoamine oxidase-B (MAO-B) and imidazoline2 binding site (I2BS), which are predominantly expressed in the mitochondrial membranes of astrocytes and are upregulated in various neurodegenerative conditions. PET tracers targeting these two MAO-B and I2BS have been evaluated in humans. [18F]THK-5351, which was originally designed to target tau aggregates in AD, showed high affinity for MAO-B and clearly visualized reactive astrocytes in progressive supranuclear palsy (PSP). However, the lack of selectivity of [18F]THK-5351 binding to both MAO-B and tau, severely limits its clinical utility as a biomarker. Recently, [18F]SMBT-1 was developed as a selective and reversible MAO-B PET tracer via compound optimization of [18F]THK-5351. In this review, we summarize the strategy underlying molecular imaging of reactive astrogliosis and clinical studies using MAO-B and I2BS PET tracers.
Keywords: MAO-B; PET; imidazoline2 binding site; radiotracers; reactive astrogliosis.
Copyright © 2022 Harada, Furumoto, Kudo, Yanai, Villemagne and Okamura.
Conflict of interest statement
NO and YK hold stocks in CLINO Co., Ltd. RH, SF, and NO were scientific consultants for CLINO Co., Ltd. The authors declare that this study received funding from Clino Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Figures

References
-
- Azzaro A. J., Ziemniak J., Kemper E., Campbell B. J., Vandenberg C. (2007). Pharmacokinetics and absolute bioavailability of selegiline following treatment of healthy subjects with the selegiline transdermal system (6 mg/24 h): a comparison with oral selegiline capsules. J. Clin. Pharmacol. 47 1256–1267. 10.1177/0091270007304779 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

