Asiatic Acid Attenuates Osteoporotic Bone Loss in Ovariectomized Mice Through Inhibiting NF-kappaB/MAPK/ Protein Kinase B Signaling Pathway
- PMID: 35211021
- PMCID: PMC8861314
- DOI: 10.3389/fphar.2022.829741
Asiatic Acid Attenuates Osteoporotic Bone Loss in Ovariectomized Mice Through Inhibiting NF-kappaB/MAPK/ Protein Kinase B Signaling Pathway
Abstract
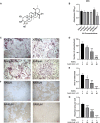
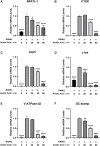
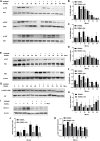
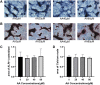
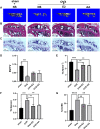
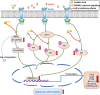
Osteoporosis is a condition associated with osteolytic bone disease that is primarily characterized by inordinate osteoclast activation. Protein kinase B (Akt) pathways activated by receptor activator of nuclear factor kappa-B ligand (RANKL) are essential for osteoclastogenesis. Asiatic acid (AA) is a natural pentacyclic triterpenoid compound extracted from a traditional Chinese herb that exhibits a wide range of biological activities. AA has been found to alleviate the hypertrophic and fibrotic phenotype of chondrocytes via the Akt signaling pathway. In this study, we investigated whether AA alleviated bone loss by inhibiting the Akt signaling pathway during osteoclastogenesis and its effect on osteoblasts. The effect of AA cytotoxicity on mouse bone marrow-derived macrophages/monocytes (BMMs) was evaluated in vitro using a Cell Counting Kit-8 assay. The effects of AA on osteoclast differentiation and function were detected using tartrate-resistant acid phosphatase (TRAP) staining and a pit formation assay. A Western blot and qRT-PCR were conducted to evaluate the expression of osteoclast-specific genes and protein signaling molecules. In addition, alkaline phosphatase and alizarin red staining were performed to assess osteoblast differentiation and mineralization. The bone protective effect of AA was investigated in vivo using ovariectomized mice. we found that AA could dose-dependently inhibit RANKL-induced osteoclastogenesis. Moreover, the pit formation assay revealed that osteoclast function was suppressed by treatment with AA. Moreover, the expression of osteoclast-specific genes was found to be substantially decreased during osteoclastogenesis. Analysis of the molecular mechanisms showed that AA could inhibit NF-kappaB/MAPK/Akt signaling pathway, as well as the downstream factors of NFATc1 in the osteoclast signaling pathway activated by RANKL. However, AA did not significantly promote osteoblast differentiation and mineralization. The in vivo experiments suggested that AA could alleviate ovariectomy-induced bone loss in ovariectomized mice. Our results demonstrate that AA can inhibit osteoclastogenesis and prevent ovariectomy-induced bone loss by inhibiting the NF-kappaB/MAPK/Akt signaling pathway. The discovery of the new molecular mechanism that AA inhibits osteoclastogenesis provides essential evidence to support the use of AA as a potential drug for the treatment of osteoclast-related diseases.
Keywords: asiatic acid; osteoblast; osteoclastogenesis; osteoporosis; protein kinase B.
Copyright © 2022 Dong, Zeng, Yang, Qiu and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Rosavin suppresses osteoclastogenesis in vivo and in vitro by blocking the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways.Ann Transl Med. 2021 Mar;9(5):383. doi: 10.21037/atm-20-4255. Ann Transl Med. 2021. PMID: 33842604 Free PMC article.
-
Leonurine hydrochloride inhibits osteoclastogenesis and prevents osteoporosis associated with estrogen deficiency by inhibiting the NF-κB and PI3K/Akt signaling pathways.Bone. 2015 Jun;75:128-37. doi: 10.1016/j.bone.2015.02.017. Epub 2015 Feb 21. Bone. 2015. PMID: 25708053
-
Caffeic acid 3,4-dihydroxy-phenethyl ester suppresses receptor activator of NF-κB ligand–induced osteoclastogenesis and prevents ovariectomy-induced bone loss through inhibition of mitogen-activated protein kinase/activator protein 1 and Ca2+–nuclear factor of activated T-cells cytoplasmic 1 signaling pathways.J Bone Miner Res. 2012 Jun;27(6):1298-1308. doi: 10.1002/jbmr.1576. J Bone Miner Res. 2012. PMID: 22337253
-
The role of lactoferrin in bone remodeling: evaluation of its potential in targeted delivery and treatment of metabolic bone diseases and orthopedic conditions.Front Endocrinol (Lausanne). 2023 Aug 23;14:1218148. doi: 10.3389/fendo.2023.1218148. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37680888 Free PMC article. Review.
-
Marine natural products that inhibit osteoclastogenesis and promote osteoblast differentiation.J Nat Med. 2022 Jun;76(3):575-583. doi: 10.1007/s11418-022-01622-5. Epub 2022 Apr 10. J Nat Med. 2022. PMID: 35397769 Free PMC article. Review.
Cited by
-
Synergistic Combination of Luteolin and Asiatic Acid on Cervical Cancer In Vitro and In Vivo.Cancers (Basel). 2023 Jan 16;15(2):548. doi: 10.3390/cancers15020548. Cancers (Basel). 2023. PMID: 36672499 Free PMC article.
-
Protaetia brevitarsis Extract Attenuates RANKL-Induced Osteoclastogenesis by Inhibiting the JNK/NF-κB/PLCγ2 Signaling Pathway.Nutrients. 2023 Jul 19;15(14):3193. doi: 10.3390/nu15143193. Nutrients. 2023. PMID: 37513611 Free PMC article.
-
Unveiling the potential of Butylphthalide: inhibiting osteoclastogenesis and preventing bone loss.Front Pharmacol. 2024 Feb 23;15:1347241. doi: 10.3389/fphar.2024.1347241. eCollection 2024. Front Pharmacol. 2024. PMID: 38464734 Free PMC article.
-
Chrysoeriol: a natural RANKL inhibitor targeting osteoclastogenesis and ROS regulation for osteoporosis therapy.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jun;398(6):7389-7407. doi: 10.1007/s00210-024-03714-3. Epub 2025 Jan 4. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39755833
-
Periplogenin attenuates LPS-mediated inflammatory osteolysis through the suppression of osteoclastogenesis via reducing the NF-κB and MAPK signaling pathways.Cell Death Discov. 2024 Feb 17;10(1):86. doi: 10.1038/s41420-024-01856-0. Cell Death Discov. 2024. PMID: 38368392 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

