Long-Term Improvement in a Chinese Cohort of Glucocorticoid-Resistant Childhood-Onset Myasthenia Gravis Patients Treated With Tacrolimus
- PMID: 35211085
- PMCID: PMC8860838
- DOI: 10.3389/fneur.2022.820205
Long-Term Improvement in a Chinese Cohort of Glucocorticoid-Resistant Childhood-Onset Myasthenia Gravis Patients Treated With Tacrolimus
Abstract
Objectives: To evaluate the long-term outcome of tacrolimus for childhood-onset myasthenia gravis (CMG) with an inadequate response to glucocorticoids, and investigate factors associated with favorable outcomes following tacrolimus treatment.
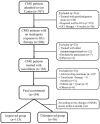
Methods: A retrospective, observational cohort study was performed for CMG patients who had not improved satisfactorily after sufficient prednisone therapy for at least 8 weeks. All patients were given tacrolimus in doses of 2-3 mg for more than 6 months. The primary efficacy outcome was assessed using the prednisone dose, quantitative MG (QMG), and MG-activity of daily living (ADL) scores. The participants were divided into improved and unimproved groups based on changes in QMG scores to investigate the risk factors that affected tacrolimus efficacy.
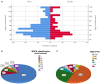
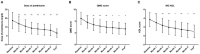
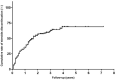
Results: A total of 149 glucocorticoid resistant CMG patients were finally enrolled in our study, with 113 (75.8%) responding well to tacrolimus (defined as minimal manifestation status or better). One month after initiating tacrolimus, there was a noticeable improvement in prednisone dose, QMG, and ADL scores, which continued to improve throughout the study. More importantly, the prednisone was eventually stopped in 89 of the patients (78.8%). Thymus type [odds ratio (OR) = 3.156, 95% confidence interval (CI) 1.427-6.978; P = 0.005] and pre-intervention status (OR = 0.284, 95%CI 0.109-0.741; P = 0.010) were independent predictors of tacrolimus efficacy after controlling for confounding factors in multiple logistic regression.
Conclusion: The majority of glucocorticoid-resistant CMG patients have a good long-term prognosis after adding tacrolimus. Thymus type and pre-intervention status can serve as potential predictors affecting the efficacy of tacrolimus.
Keywords: children; myasthenia gravis; pre-intervention status; tacrolimus; thymus type.
Copyright © 2022 Bi, Cao, Lin, Zhang, Liu, Gui and Bu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Remission and relapses of myasthenia gravis on long-term tacrolimus: a retrospective cross-sectional study of a Chinese cohort.Ther Adv Chronic Dis. 2022 Sep 6;13:20406223221122538. doi: 10.1177/20406223221122538. eCollection 2022. Ther Adv Chronic Dis. 2022. PMID: 36093262 Free PMC article.
-
Long-term efficacy and safety of tacrolimus in young children with myasthenia gravis.J Clin Neurosci. 2023 Oct;116:93-98. doi: 10.1016/j.jocn.2023.08.022. Epub 2023 Sep 3. J Clin Neurosci. 2023. PMID: 37669613
-
Tacrolimus in the treatment of myasthenia gravis in patients with an inadequate response to glucocorticoid therapy: randomized, double-blind, placebo-controlled study conducted in China.Ther Adv Neurol Disord. 2017 Sep;10(9):315-325. doi: 10.1177/1756285617721092. Epub 2017 Jul 26. Ther Adv Neurol Disord. 2017. PMID: 28861121 Free PMC article.
-
Long-term efficacy and safety of leflunomide combined with low-dose prednisone in treatment of myasthenia gravis: a retrospective study.Acta Neurol Belg. 2024 Feb;124(1):175-182. doi: 10.1007/s13760-023-02367-y. Epub 2023 Sep 1. Acta Neurol Belg. 2024. PMID: 37656361 Review.
-
Efficacy and Safety of Tacrolimus in Myasthenia Gravis: A Systematic Review and Meta-analysis.Ann Indian Acad Neurol. 2017 Oct-Dec;20(4):341-347. doi: 10.4103/aian.AIAN_97_17. Ann Indian Acad Neurol. 2017. PMID: 29184334 Free PMC article. Review.
Cited by
-
Tacrolimus Combined with Corticosteroids Improved the Outcome of CIDP Patients with Autoantibodies Against Paranodal Proteins.Neuropsychiatr Dis Treat. 2022 Jun 16;18:1207-1217. doi: 10.2147/NDT.S361461. eCollection 2022. Neuropsychiatr Dis Treat. 2022. PMID: 35734550 Free PMC article.
-
Remission and relapses of myasthenia gravis on long-term tacrolimus: a retrospective cross-sectional study of a Chinese cohort.Ther Adv Chronic Dis. 2022 Sep 6;13:20406223221122538. doi: 10.1177/20406223221122538. eCollection 2022. Ther Adv Chronic Dis. 2022. PMID: 36093262 Free PMC article.
-
Long-term efficacy and safety of tacrolimus in anti-MuSK antibody-positive myasthenia gravis: a retrospective single-center cohort study.Neurol Sci. 2025 Feb;46(2):943-949. doi: 10.1007/s10072-024-07819-8. Epub 2024 Nov 6. Neurol Sci. 2025. PMID: 39503950
-
Treatment of juvenile myasthenia gravis with tacrolimus: A cohort study.Eur J Neurol. 2024 Dec;31(12):e16466. doi: 10.1111/ene.16466. Epub 2024 Sep 4. Eur J Neurol. 2024. PMID: 39230556 Free PMC article.
References
LinkOut - more resources
Full Text Sources

