Novel Nanotechnological Approaches for Targeting Dorsal Root Ganglion (DRG) in Mitigating Diabetic Neuropathic Pain (DNP)
- PMID: 35211091
- PMCID: PMC8862660
- DOI: 10.3389/fendo.2021.790747
Novel Nanotechnological Approaches for Targeting Dorsal Root Ganglion (DRG) in Mitigating Diabetic Neuropathic Pain (DNP)
Abstract
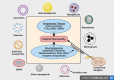
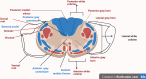
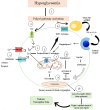
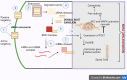
Diabetic neuropathy is the most entrenched complication of diabetes. Usually, it affects the distal foot and toes, which then gradually approaches the lower part of the legs. Diabetic foot ulcer (DFU) could be one of the worst complications of diabetes mellitus. Long-term diabetes leads to hyperglycemia, which is the utmost contributor to neuropathic pain. Hyperglycemia causing an upregulation of voltage-gated sodium channels in the dorsal root ganglion (DRG) was often observed in models of neuropathic pain. DRG opening frequency increases intracellular sodium ion levels, which further causes increased calcium channel opening and stimulates other pathways leading to diabetic peripheral neuropathy (DPN). Currently, pain due to diabetic neuropathy is managed via antidepressants, opioids, gamma-aminobutyric acid (GABA) analogs, and topical agents such as capsaicin. Despite the availability of various treatment strategies, the percentage of patients achieving adequate pain relief remains low. Many factors contribute to this condition, such as lack of specificity and adverse effects such as light-headedness, languidness, and multiple daily doses. Therefore, nanotechnology outperforms in every aspect, providing several benefits compared to traditional therapy such as site-specific and targeted drug delivery. Nanotechnology is the branch of science that deals with the development of nanoscale materials and products, even smaller than 100 nm. Carriers can improve their efficacy with reduced side effects by incorporating drugs into the novel delivery systems. Thus, the utilization of nanotechnological approaches such as nanoparticles, polymeric nanoparticles, inorganic nanoparticles, lipid nanoparticles, gene therapy (siRNA and miRNA), and extracellular vesicles can extensively contribute to relieving neuropathic pain.
Keywords: diabetic neuropathic pain (DNP); dorsal root ganglion (DRG); extracellular vesicles; ligand-based targeting; nanoparticles; nanotechnology; siRNA.
Copyright © 2022 Bhandari, Sharma and Kuhad.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

