Negative Regulation of the IL-1 System by IL-1R2 and IL-1R8: Relevance in Pathophysiology and Disease
- PMID: 35211118
- PMCID: PMC8861086
- DOI: 10.3389/fimmu.2022.804641
Negative Regulation of the IL-1 System by IL-1R2 and IL-1R8: Relevance in Pathophysiology and Disease
Abstract
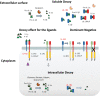
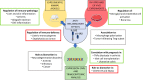
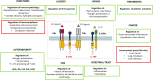
Interleukin-1 (IL-1) is a primary cytokine of innate immunity and inflammation. IL-1 belongs to a complex family including ligands with agonist activity, receptor antagonists, and an anti-inflammatory cytokine. The receptors for these ligands, the IL-1 Receptor (IL-1R) family, include signaling receptor complexes, decoy receptors, and negative regulators. Agonists and regulatory molecules co-evolved, suggesting the evolutionary relevance of a tight control of inflammatory responses, which ensures a balance between amplification of innate immunity and uncontrolled inflammation. IL-1 family members interact with innate immunity cells promoting innate immunity, as well as with innate and adaptive lymphoid cells, contributing to their differentiation and functional polarization and plasticity. Here we will review the properties of two key regulatory receptors of the IL-1 system, IL-1R2, the first decoy receptor identified, and IL-1R8, a pleiotropic regulator of different IL-1 family members and co-receptor for IL-37, the anti-inflammatory member of the IL-1 family. Their complex impact in pathology, ranging from infections and inflammatory responses, to cancer and neurologic disorders, as well as clinical implications and potential therapeutic exploitation will be presented.
Keywords: inflammation; innate immunity; interleukin 1; negative regulation; toll-like-receptors.
Copyright © 2022 Supino, Minute, Mariancini, Riva, Magrini and Garlanda.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Tuning inflammation and immunity by the negative regulators IL-1R2 and IL-1R8.Immunol Rev. 2018 Jan;281(1):233-247. doi: 10.1111/imr.12609. Immunol Rev. 2018. PMID: 29247989 Free PMC article. Review.
-
The interleukin-1 family: back to the future.Immunity. 2013 Dec 12;39(6):1003-18. doi: 10.1016/j.immuni.2013.11.010. Immunity. 2013. PMID: 24332029 Free PMC article. Review.
-
The family of the interleukin-1 receptors.Immunol Rev. 2018 Jan;281(1):197-232. doi: 10.1111/imr.12606. Immunol Rev. 2018. PMID: 29248002 Review.
-
IL-1R8: A molecular brake of anti-tumor and anti-viral activity of NK cells and ILC.Semin Immunol. 2023 Mar;66:101712. doi: 10.1016/j.smim.2023.101712. Epub 2023 Feb 6. Semin Immunol. 2023. PMID: 36753974 Review.
-
Negative regulatory receptors of the IL-1 family.Semin Immunol. 2013 Dec 15;25(6):408-15. doi: 10.1016/j.smim.2013.10.019. Epub 2013 Nov 14. Semin Immunol. 2013. PMID: 24239046 Review.
Cited by
-
Decoy Receptors Regulation by Resveratrol in Lipopolysaccharide-Activated Microglia.Cells. 2023 Feb 21;12(5):681. doi: 10.3390/cells12050681. Cells. 2023. PMID: 36899817 Free PMC article.
-
Novel insights into IL-37: an anti-inflammatory cytokine with emerging roles in anti-cancer process.Front Immunol. 2023 Oct 20;14:1278521. doi: 10.3389/fimmu.2023.1278521. eCollection 2023. Front Immunol. 2023. PMID: 37928545 Free PMC article. Review.
-
IL-1 Superfamily Across 400+ Species: Therapeutic Targets and Disease Implications.Biology (Basel). 2025 May 17;14(5):561. doi: 10.3390/biology14050561. Biology (Basel). 2025. PMID: 40427750 Free PMC article.
-
From periphery to center stage: 50 years of advancements in innate immunity.Cell. 2024 Apr 25;187(9):2030-2051. doi: 10.1016/j.cell.2024.03.036. Cell. 2024. PMID: 38670064 Free PMC article. Review.
-
Soluble interleukin-1 receptor type 2 plasma levels in Parkinson's disease: relationship with cardiac autonomic profile before and after peripheral mechanical somatosensory stimulation.Front Physiol. 2023 Aug 16;14:1168652. doi: 10.3389/fphys.2023.1168652. eCollection 2023. Front Physiol. 2023. PMID: 37664433 Free PMC article.

