Chloroquine Suppresses Effector B-Cell Functions and Has Differential Impact on Regulatory B-Cell Subsets
- PMID: 35211119
- PMCID: PMC8860819
- DOI: 10.3389/fimmu.2022.818704
Chloroquine Suppresses Effector B-Cell Functions and Has Differential Impact on Regulatory B-Cell Subsets
Abstract
Objectives: Chloroquine (CQ) is approved for treatment of B-cell mediated diseases such as rheumatoid arthritis and systemic lupus erythematosus. However, the exact mode of action in these diseases has not been studied and it remains unclear which effect CQ has on B-cells. Thus, it was the aim of this study to investigate to which extent CQ affects functionality of effector and regulatory B-cell.
Methods: For this purpose, B-cells were isolated from peripheral blood of healthy controls and renal transplant patients. B-cells were stimulated in presence or absence of CQ and Interleukin-10 (IL-10) and Granzyme B (GrB) secretion were assessed. In addition, effector functions such as plasma cell formation, and Immunoglobulin G (IgG) secretion were studied.
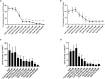
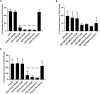
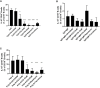
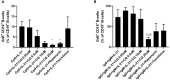
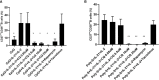
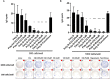
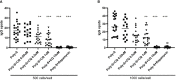
Results: CQ suppressed Toll-Like-Receptor (TLR)-9 induced B-cell proliferation in a dose-dependent manner. IL-10pos regulatory B-cells were suppressed by CQ already at low concentrations whereas anti-IgG/IgM-induced GrB secreting regulatory B-cells were less susceptible. Plasma blast formation and IgG secretion was potently suppressed by CQ. Moreover, purified B-cells from renal transplant patients were also susceptible to CQ-induced suppression of effector B-cell functions as observed by diminished IgG secretion.
Conclusion: In conclusion, CQ had a suppressive effect on IL-10 regulatory B-cells whereas GrB secreting regulatory B-cells were less affected. Effector functions of B-cells such as plasma blast formation and IgG secretion were also inhibited by CQ. Effector B-cells derived from renal transplant patients already under immunosuppression could be suppressed by CQ. These findings may partly explain the clinical efficacy of CQ in B-cell mediated autoimmune diseases. The application of CQ in other disease contexts where suppression of effector B-cells could offer a benefit, such as renal transplantation, may hypothetically be advantageous.
Keywords: B-cells; chloroquine; effector B-cells; regulatory B (Breg) cells; renal transplantation.
Copyright © 2022 Ma, Dai, Witzke, Xu, Lindemann, Kribben, Dolff and Wilde.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Interleukin 21-induced granzyme B-expressing B cells infiltrate tumors and regulate T cells.Cancer Res. 2013 Apr 15;73(8):2468-79. doi: 10.1158/0008-5472.CAN-12-3450. Epub 2013 Feb 5. Cancer Res. 2013. PMID: 23384943
-
Farnesyltransferase-inhibitors exert in vitro immunosuppressive capacity by inhibiting human B-cells.Front Transplant. 2023 Nov 9;2:1233322. doi: 10.3389/frtra.2023.1233322. eCollection 2023. Front Transplant. 2023. PMID: 38993912 Free PMC article.
-
Granzyme B producing regulatory B-cells in patients with giant cell arteritis.Clin Exp Rheumatol. 2025 Apr;43(4):655-660. doi: 10.55563/clinexprheumatol/37o5o5. Epub 2025 Mar 14. Clin Exp Rheumatol. 2025. PMID: 40095634
-
B lymphocyte function in patients with rheumatoid arthritis: impact of regulatory T lymphocytes and macrophages--modulation by antirheumatic drugs.Dan Med Bull. 1988 Apr;35(2):140-57. Dan Med Bull. 1988. PMID: 3282810 Review.
-
Elucidating the Pivotal Immunomodulatory and Anti-Inflammatory Potentials of Chloroquine and Hydroxychloroquine.J Immunol Res. 2020 Sep 25;2020:4582612. doi: 10.1155/2020/4582612. eCollection 2020. J Immunol Res. 2020. PMID: 33062720 Free PMC article. Review.
Cited by
-
Mechanisms Underlying the Effects of Chloroquine on Red Blood Cells Metabolism.Int J Mol Sci. 2024 Jun 11;25(12):6424. doi: 10.3390/ijms25126424. Int J Mol Sci. 2024. PMID: 38928131 Free PMC article.
-
Revealing the role of regulatory b cells in cancer: development, function and treatment significance.Cancer Immunol Immunother. 2025 Feb 25;74(4):125. doi: 10.1007/s00262-025-03973-w. Cancer Immunol Immunother. 2025. PMID: 39998678 Free PMC article. Review.
-
Autoimmunity and Carcinogenesis: Their Relationship under the Umbrella of Autophagy.Biomedicines. 2023 Apr 8;11(4):1130. doi: 10.3390/biomedicines11041130. Biomedicines. 2023. PMID: 37189748 Free PMC article. Review.
-
Effector and regulatory B-cell imbalance in systemic sclerosis: cooperation or competition?Clin Rheumatol. 2024 Sep;43(9):2783-2789. doi: 10.1007/s10067-024-07086-0. Epub 2024 Jul 30. Clin Rheumatol. 2024. PMID: 39080112 Free PMC article. Review.
-
Immunosuppression by hydroxychloroquine: mechanistic proof in in vitro experiments but limited systemic activity in a randomized placebo-controlled clinical pharmacology study.Immunol Res. 2023 Aug;71(4):617-627. doi: 10.1007/s12026-023-09367-3. Epub 2023 Feb 22. Immunol Res. 2023. PMID: 36811819 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

