Cholinergic blockade of neuroinflammation: from tissue to RNA regulators
- PMID: 35211331
- PMCID: PMC8837817
- DOI: 10.1042/NS20210035
Cholinergic blockade of neuroinflammation: from tissue to RNA regulators
Abstract
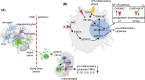
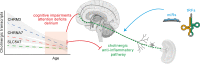
Inflammatory stimuli and consequent pro-inflammatory immune responses may facilitate neurodegeneration and threaten survival following pathogen infection or trauma, but potential controllers preventing these risks are incompletely understood. Here, we argue that small RNA regulators of acetylcholine (ACh) signaling, including microRNAs (miRs) and transfer RNA fragments (tRFs) may tilt the balance between innate and adaptive immunity, avoid chronic inflammation and prevent the neuroinflammation-mediated exacerbation of many neurological diseases. While the restrictive permeability of the blood-brain barrier (BBB) protects the brain from peripheral immune events, this barrier can be disrupted by inflammation and is weakened with age. The consequently dysregulated balance between pro- and anti-inflammatory processes may modify the immune activities of brain microglia, astrocytes, perivascular macrophages, oligodendrocytes and dendritic cells, leading to neuronal damage. Notably, the vagus nerve mediates the peripheral cholinergic anti-inflammatory reflex and underlines the consistent control of body-brain inflammation by pro-inflammatory cytokines, which affect cholinergic functions; therefore, the disruption of this reflex can exacerbate cognitive impairments such as attention deficits and delirium. RNA regulators can contribute to re-balancing the cholinergic network and avoiding its chronic deterioration, and their activities may differ between men and women and/or wear off with age. This can lead to hypersensitivity of aged patients to inflammation and higher risks of neuroinflammation-driven cholinergic impairments such as delirium and dementia following COVID-19 infection. The age- and sex-driven differences in post-transcriptional RNA regulators of cholinergic elements may hence indicate new personalized therapeutic options for neuroinflammatory diseases.
Keywords: acetylcholine; aging; cholinergic; microRNA; neuroinflammation; sex.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

