A Whole-Body Musculoskeletal Model of the Mouse
- PMID: 35211364
- PMCID: PMC8865483
- DOI: 10.1109/access.2021.3133078
A Whole-Body Musculoskeletal Model of the Mouse
Abstract
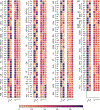
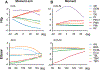
Neural control of movement cannot be fully understood without careful consideration of interactions between the neural and biomechanical components. Recent advancements in mouse molecular genetics allow for the identification and manipulation of constituent elements underlying the neural control of movement. To complement experimental studies and investigate the mechanisms by which the neural circuitry interacts with the body and the environment, computational studies modeling motor behaviors in mice need to incorporate a model of the mouse musculoskeletal system. Here, we present the first fully articulated musculoskeletal model of the mouse. The mouse skeletal system has been developed from anatomical references and includes the sets of bones in all body compartments, including four limbs, spine, head and tail. Joints between all bones allow for simulation of full 3D mouse kinematics and kinetics. Hindlimb and forelimb musculature has been implemented using Hill-type muscle models. We analyzed the mouse whole-body model and described the moment-arms for different hindlimb and forelimb muscles, the moments applied by these muscles on the joints, and their involvement in limb movements at different limb/body configurations. The model represents a necessary step for the subsequent development of a comprehensive neuro-biomechanical model of freely behaving mice; this will close the loop between the neural control and the physical interactions between the body and the environment.
Keywords: Mouse; biomechanical; biomechanics; moment-arms; musculoskeletal; neuromechanical; open-source model.
Figures

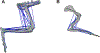
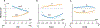



References
-
- Grillner S. and Manira AE, “Current principles of motor control, with special reference to vertebrate locomotion,” Physiol. Rev, vol. 100, no. 1, pp. 271–320, Jan. 2020. - PubMed
-
- Grillner S, “Control of locomotion in bipeds, tetrapods, and fish,” in Comprehensive Physiology. Atlanta, GA, USA: American Cancer Society, 2011, pp. 1179–1236.
Grants and funding
LinkOut - more resources
Full Text Sources
