Gastrointestinal Autonomic Neuropathy Exacerbates Gut Microbiota Dysbiosis in Adult Patients With Type 2 Diabetes Mellitus
- PMID: 35211420
- PMCID: PMC8861497
- DOI: 10.3389/fcimb.2021.804733
Gastrointestinal Autonomic Neuropathy Exacerbates Gut Microbiota Dysbiosis in Adult Patients With Type 2 Diabetes Mellitus
Abstract
Objective: The diabetic autonomic neuropathy is one of the most common complications in type 2 diabetes mellitus (T2DM), especially gastrointestinal autonomic neuropathy (GAN), which occurs in up to 75% of patients. The study aimed to investigate the gut microbiota composition, structure, and function in T2DM patients with GAN (T2DM_GAN) and set up a link between gut microbiota and clinical characteristics of patients.
Methods: DNA was extracted from fecal samples of three groups using the kit method: healthy volunteers (n = 19), the patients with T2DM (n = 76), and T2DM_GAN (n = 27). Sequencing of 16S ribosomal DNA was performed using the MiSeq platform.
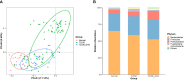
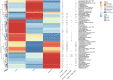
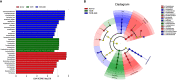
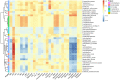
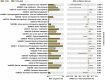
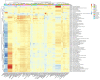
Results: According to the clinical data, higher age, lower triglyceride, and lower body mass index were the main features of patients with T2DM_GAN. The gut microbiota analysis showed that Bacteroidetes, Firmicutes, and Proteobacteria constituted the three dominant phyla in healthy individuals. In addition, the gut microbiota structure and function of T2DM_GAN patients were clearly different from that of T2DM patients. T2DM patients were characterized by Fusobacteria, Fusobacteriia, Fusobacteriales, Fusobacteriaceae, Fusobacterium, Lachnoclostridium, and Fusobacterium_mortiferum. Those gut microbiota may be involved in carotenoid and flavonoid biosyntheses. Relatively, the Gammaproteobacteria, Enterobacteriales, Enterobacteriaceae, Escherichia-Shigella, Megasphaera, Escherichia_coli, and Megasphaera_elsdenii were characteristic in the T2DM_GAN patients. Those may be involved in bacterial invasion of epithelial cells and pathogenic Escherichia coli infection.
Conclusions: GAN exacerbated gut microbiota dysbiosis in adult patients with T2DM. The findings indicated that phyla Fusobacteria and class Gammaproteobacteria were closely related to the occurrence of T2DM. Especially the latter may promote T2DM_GAN.
Keywords: diagnosis; gastrointestinal autonomic neuropathy; gastrointestinal symptoms; gut microbiota; type 2 diabetes mellitus.
Copyright © 2022 Du, Neng, Li, Kang, Guo, Huang, Chen, Yang, Hong, Zhou, Zhao, Yu, Su and Kong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Alterations of gut microbiome in patients with type 2 diabetes mellitus who had undergone cholecystectomy.Am J Physiol Endocrinol Metab. 2021 Jan 1;320(1):E113-E121. doi: 10.1152/ajpendo.00471.2020. Epub 2020 Nov 9. Am J Physiol Endocrinol Metab. 2021. PMID: 33166187
-
Characteristics of gut microbiota in adult patients with type 1 and type 2 diabetes based on next‑generation sequencing of the 16S rRNA gene fragment.Pol Arch Intern Med. 2018 Jun 30;128(6):336-343. doi: 10.20452/pamw.4246. Epub 2018 Apr 15. Pol Arch Intern Med. 2018. PMID: 29657308
-
Ethnicity-matched case-control study reveals significant gut microbiota differences in Malaysian adults with type 2 diabetes.J Med Microbiol. 2025 Jan;74(1). doi: 10.1099/jmm.0.001963. J Med Microbiol. 2025. PMID: 39886920
-
Characteristics of the gut microbiome in women with gestational diabetes mellitus: A systematic review.PLoS One. 2022 Jan 13;17(1):e0262618. doi: 10.1371/journal.pone.0262618. eCollection 2022. PLoS One. 2022. PMID: 35025980 Free PMC article.
-
A systematic review on gut microbiota in type 2 diabetes mellitus.Front Endocrinol (Lausanne). 2025 Jan 17;15:1486793. doi: 10.3389/fendo.2024.1486793. eCollection 2024. Front Endocrinol (Lausanne). 2025. PMID: 39897957 Free PMC article.
Cited by
-
Type-2 Diabetes Mellitus and the Gut Microbiota: Systematic Review.Cureus. 2023 Nov 30;15(11):e49740. doi: 10.7759/cureus.49740. eCollection 2023 Nov. Cureus. 2023. PMID: 38161953 Free PMC article. Review.
-
Stress and depression-associated shifts in gut microbiota: A pilot study of human pregnancy.Brain Behav Immun Health. 2024 Jan 26;36:100730. doi: 10.1016/j.bbih.2024.100730. eCollection 2024 Mar. Brain Behav Immun Health. 2024. PMID: 38323225 Free PMC article.
-
Yi-Shen-Hua-Shi regulates intestinal microbiota dysbiosis and protects against proteinuria in patients with chronic kidney disease: a randomized controlled study.Pharm Biol. 2024 Dec;62(1):356-366. doi: 10.1080/13880209.2024.2345080. Epub 2024 May 9. Pharm Biol. 2024. PMID: 38720666 Free PMC article. Clinical Trial.
-
Genome sequencing of Escherichia coli phage UFJF_EcSW4 reveals a novel lytic Kayfunavirus species.3 Biotech. 2025 Jan;15(1):10. doi: 10.1007/s13205-024-04172-7. Epub 2024 Dec 15. 3 Biotech. 2025. PMID: 39691801
-
Microbiome and metabolic disorder in prolactinoma: intrinsic gender differences and extrinsic therapy effects.Pituitary. 2025 Jul 3;28(4):83. doi: 10.1007/s11102-025-01544-x. Pituitary. 2025. PMID: 40608175 No abstract available.
References
-
- Asgharnezhad M., Joukar F., Fathalipour M., Khosousi M., Hassanipour S., Pourshams A., et al. . (2019). Gastrointestinal Symptoms in Patients With Diabetes Mellitus and Non-Diabetic: A Cross-Sectional Study in North of Iran. Diabetes Metab. Syndr. 13 (3), 2236–2240. doi: 10.1016/j.dsx.2019.05.028 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

