Supraspinal Mechanisms Underlying Ocular Pain
- PMID: 35211480
- PMCID: PMC8862711
- DOI: 10.3389/fmed.2021.768649
Supraspinal Mechanisms Underlying Ocular Pain
Abstract
Supraspinal mechanisms of pain are increasingly understood to underlie neuropathic ocular conditions previously thought to be exclusively peripheral in nature. Isolating individual causes of centralized chronic conditions and differentiating them is critical to understanding the mechanisms underlying neuropathic eye pain and ultimately its treatment. Though few functional imaging studies have focused on the eye as an end-organ for the transduction of noxious stimuli, the brain networks related to pain processing have been extensively studied with functional neuroimaging over the past 20 years. This article will review the supraspinal mechanisms that underlie pain as they relate to the eye.
Keywords: brain; brainstem; eye; fMRI; neuroimaging; ocular; pain; supraspinal.
Copyright © 2022 Pondelis and Moulton.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



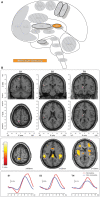

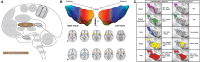


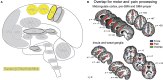

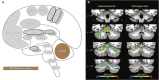

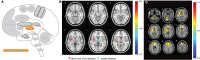
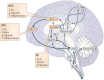
Similar articles
-
Review of neuroimaging studies related to pain modulation.Scand J Pain. 2018 Jul 1;2(3):108-120. doi: 10.1016/j.sjpain.2011.05.005. Scand J Pain. 2018. PMID: 29913745
-
Nociceptive behavior in animal models for peripheral neuropathy: spinal and supraspinal mechanisms.Prog Neurobiol. 2008 Sep;86(1):22-47. doi: 10.1016/j.pneurobio.2008.06.002. Epub 2008 Jun 18. Prog Neurobiol. 2008. PMID: 18602968 Review.
-
Supraspinal characterization of the thermal grill illusion with fMRI.Mol Pain. 2014 Mar 11;10:18. doi: 10.1186/1744-8069-10-18. Mol Pain. 2014. PMID: 24612493 Free PMC article.
-
Evaluation of a magnetic resonance-compatible dentoalveolar tactile stimulus device.BMC Neurosci. 2010 Oct 28;11:142. doi: 10.1186/1471-2202-11-142. BMC Neurosci. 2010. PMID: 21029454 Free PMC article.
-
Burning Eye Syndrome: Do Neuropathic Pain Mechanisms Underlie Chronic Dry Eye?Pain Med. 2016 Apr;17(4):746-55. doi: 10.1093/pm/pnv070. Epub 2015 Dec 24. Pain Med. 2016. PMID: 26814296 Free PMC article. Review.
Cited by
-
Disentangling the neurological basis of chronic ocular pain using clinical, self-report, and brain imaging data: use of K-means clustering to explore patient phenotypes.Front Neurol. 2023 Nov 16;14:1265082. doi: 10.3389/fneur.2023.1265082. eCollection 2023. Front Neurol. 2023. PMID: 38033775 Free PMC article.
References
-
- Melzack R, Casey KL. Sensory, motivational, and central control determinants of pain: a new conceptual model. The skin senses. (1968) 1:423–43.
Publication types
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

