Inteins as Drug Targets and Therapeutic Tools
- PMID: 35211511
- PMCID: PMC8861304
- DOI: 10.3389/fmolb.2022.821146
Inteins as Drug Targets and Therapeutic Tools
Abstract
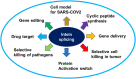
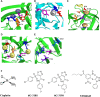
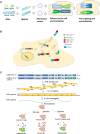
Multidrug-resistant pathogens are of significant concern in recent years. Hence new antifungal and anti-bacterial drug targets are urgently needed before the situation goes beyond control. Inteins are polypeptides that self-splice from exteins without the need for cofactors or external energy, resulting in joining of extein fragments. Inteins are present in many organisms, including human pathogens such as Mycobacterium tuberculosis, Cryptococcus neoformans, C. gattii, and Aspergillus fumigatus. Because intein elements are not present in human genes, they are attractive drug targets to develop antifungals and antibiotics. Thus far, a few inhibitors of intein splicing have been reported. Metal-ions such as Zn2+ and Cu2+, and platinum-containing compound cisplatin inhibit intein splicing in M. tuberculosis and C. neoformans by binding to the active site cysteines. A small-molecule inhibitor 6G-318S and its derivative 6G-319S are found to inhibit intein splicing in C. neoformans and C. gattii with a MIC in nanomolar concentrations. Inteins have also been used in many other applications. Intein can be used in activating a protein inside a cell using small molecules. Moreover, split intein can be used to deliver large genes in experimental gene therapy and to kill selected species in a mixed population of microbes by taking advantage of the toxin-antitoxin system. Furthermore, split inteins are used in synthesizing cyclic peptides and in developing cell culture model to study infectious viruses including SARS-CoV-2 in the biosafety level (BSL) 2 facility. This mini-review discusses the recent research developments of inteins in drug discovery and therapeutic research.
Keywords: anti-microbial; drug target; inhibitor; intein; therapeutic tool.
Copyright © 2022 Tharappel, Li and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Calcimycin Inhibits Cryptococcus neoformans In Vitro and In Vivo by Targeting the Prp8 Intein Splicing.ACS Infect Dis. 2022 Sep 9;8(9):1851-1868. doi: 10.1021/acsinfecdis.2c00137. Epub 2022 Aug 10. ACS Infect Dis. 2022. PMID: 35948057 Free PMC article.
-
Small-molecule inhibitors for the Prp8 intein as antifungal agents.Proc Natl Acad Sci U S A. 2021 Jan 12;118(2):e2008815118. doi: 10.1073/pnas.2008815118. Proc Natl Acad Sci U S A. 2021. PMID: 33397721 Free PMC article.
-
Conditional Protein Splicing Switch in Hyperthermophiles through an Intein-Extein Partnership.mBio. 2018 Jan 30;9(1):e02304-17. doi: 10.1128/mBio.02304-17. mBio. 2018. PMID: 29382734 Free PMC article.
-
Metal effect on intein splicing: A review.Biochimie. 2021 Jun;185:53-67. doi: 10.1016/j.biochi.2021.03.006. Epub 2021 Mar 13. Biochimie. 2021. PMID: 33727137 Review.
-
Intein Inhibitors as Novel Antimicrobials: Protein Splicing in Human Pathogens, Screening Methods, and Off-Target Considerations.Front Mol Biosci. 2021 Oct 7;8:752824. doi: 10.3389/fmolb.2021.752824. eCollection 2021. Front Mol Biosci. 2021. PMID: 34692773 Free PMC article. Review.
Cited by
-
Studying Peptide-Metal Ion Complex Structures by Solution-State NMR.Int J Mol Sci. 2022 Dec 15;23(24):15957. doi: 10.3390/ijms232415957. Int J Mol Sci. 2022. PMID: 36555599 Free PMC article. Review.
-
Calcimycin Inhibits Cryptococcus neoformans In Vitro and In Vivo by Targeting the Prp8 Intein Splicing.ACS Infect Dis. 2022 Sep 9;8(9):1851-1868. doi: 10.1021/acsinfecdis.2c00137. Epub 2022 Aug 10. ACS Infect Dis. 2022. PMID: 35948057 Free PMC article.
-
Inteins-mechanism of protein splicing, emerging regulatory roles, and applications in protein engineering.Front Microbiol. 2023 Nov 8;14:1305848. doi: 10.3389/fmicb.2023.1305848. eCollection 2023. Front Microbiol. 2023. PMID: 38029209 Free PMC article. Review.
-
Intein splicing efficiency and RadA levels can control the mode of archaeal DNA replication.Sci Adv. 2024 Sep 20;10(38):eadp4995. doi: 10.1126/sciadv.adp4995. Epub 2024 Sep 18. Sci Adv. 2024. PMID: 39292776 Free PMC article.
-
Optimization strategies and advances in the research and development of AAV-based gene therapy to deliver large transgenes.Clin Transl Med. 2024 Mar;14(3):e1607. doi: 10.1002/ctm2.1607. Clin Transl Med. 2024. PMID: 38488469 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

