Infectious Bronchitis Virus Infection Increases Pathogenicity of H9N2 Avian Influenza Virus by Inducing Severe Inflammatory Response
- PMID: 35211536
- PMCID: PMC8860976
- DOI: 10.3389/fvets.2021.824179
Infectious Bronchitis Virus Infection Increases Pathogenicity of H9N2 Avian Influenza Virus by Inducing Severe Inflammatory Response
Abstract
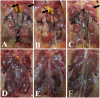
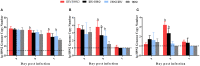
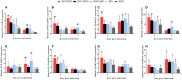
Infectious bronchitis virus (IBV) and H9N2 avian influenza virus (AIV) are frequently identified in chickens with respiratory disease. However, the role and mechanism of IBV and H9N2 AIV co-infection remain largely unknown. Specific-pathogen-free (SPF) chickens were inoculated with IBV 2 days before H9N2 virus inoculation (IBV/H9N2); with IBV and H9N2 virus simultaneously (IBV+H9N2); with H9N2 virus 2 days before IBV inoculation (H9N2/IBV); or with either IBV or H9N2 virus alone. Severe respiratory signs, pathological damage, and higher morbidity and mortality were observed in the co-infection groups compared with the IBV and H9N2 groups. In general, a higher virus load and a more intense inflammatory response were observed in the three co-infection groups, especially in the IBV/H9N2 group. The same results were observed in the transcriptome analysis of the trachea of the SPF chickens. Therefore, IBV might play a major role in the development of respiratory disease in chickens, and secondary infection with H9N2 virus further enhances the pathogenicity by inducing a severe inflammatory response. These findings may provide a reference for the prevention and control of IBV and H9N2 AIV in the poultry industry and provide insight into the molecular mechanisms of IBV and H9N2 AIV co-infection in chickens.
Keywords: H9N2 avian influenza virus; NLRP3; infectious bronchitis virus; inflammatory response; pathogenicity; transcriptome analysis.
Copyright © 2022 Kong, You, Zhang, Yuan, Xiang, Liang, Lin, Ding, Liao, Chen and Ren.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Woo PC, Lau SK, Lam CS, Lau CC, Tsang AK, Lau JH, et al. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J Virol. (2012) 86:3995–4008. 10.1128/JVI.06540-11 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

