Oral fosfomycin activity against Klebsiella pneumoniae in a dynamic bladder infection in vitro model
- PMID: 35211736
- PMCID: PMC9047678
- DOI: 10.1093/jac/dkac045
Oral fosfomycin activity against Klebsiella pneumoniae in a dynamic bladder infection in vitro model
Abstract
Introduction: The use of oral fosfomycin for urinary tract infections (UTIs) caused by non-Escherichia coli uropathogens is uncertain, including Klebsiella pneumoniae, the second most common uropathogen.
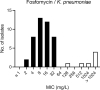
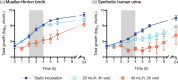
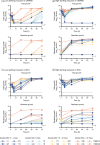
Methods: A multicompartment bladder infection in vitro model was used with standard media and synthetic human urine (SHU) to simulate urinary fosfomycin exposure after a single 3 g oral dose (fAUC0-72 16884 mg·h/L, t½ 5.5 h) against 15 K. pneumoniae isolates including ATCC 13883 (MIC 2 to >1024 mg/L) with a constant media inflow (20 mL/h) and 4-hourly voiding of each bladder. The impact of the media (CAMHB + G6P versus SHU) on fosfomycin MIC measurements, drug-free growth kinetics and regrowth after fosfomycin administration was assessed. A low and high starting inoculum (5.5 versus 7.5 log10 cfu/mL) was assessed in the bladder infection model.
Results: Compared with CAMHB, isolates in SHU had a slower growth rate doubling time (37.7 versus 24.1 min) and reduced growth capacity (9.0 ± 0.3 versus 9.4 ± 0.3 log10 cfu/mL), which was further restricted with increased inflow rate (40 mL/h) and more frequent voids (2-hourly). Regrowth was commonly observed in both media with emergence of fosfomycin resistance promoted by a high starting inoculum in CAMHB (MIC rise to ≥1024 mg/L in 13/14 isolates). Resistance was rarely detected in SHU, even with a high starting inoculum (MIC rise to ≥1024 mg/L in 2/14 isolates).
Conclusions: Simulated in an in vitro UTI model, the regrowth of K. pneumoniae urinary isolates was inadequately suppressed following oral fosfomycin therapy. Efficacy was further reduced by a high starting inoculum.
© The Author(s) 2022. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures
References
-
- Keijzers G, Macdonald SP, Udy AAet al. The Australasian Resuscitation In Sepsis Evaluation: Fluids or vasopressors in emergency department sepsis (ARISE FLUIDS), a multi-centre observational study describing current practice in Australia and New Zealand. Emerg Med Australas 2020; 32: 586–98. - PMC - PubMed
-
- Bezabih YM, Sabiiti W, Alamneh Eet al. The global prevalence and trend of human intestinal carriage of ESBL-producing Escherichia coli in the community. J Antimicrob Chemother 2021; 76: 22–9. - PubMed
-
- Quaegebeur A, Brunard L, Javaudin Fet al. Trends and prediction of antimicrobial susceptibility in urinary bacteria isolated in European emergency departments: the EuroUTI 2010–16 Study. J Antimicrob Chemother 2019; 74: 3069–76. - PubMed
-
- Vardakas KZ, Legakis NJ, Triarides Net al. Susceptibility of contemporary isolates to fosfomycin: a systematic review of the literature. Int J Antimicrob Agents 2016; 47: 269–85. - PubMed
-
- Zarakolu P, Eser OK, Otlu Bet al. In-vitro activity of fosfomycin against Escherichia coli and Klebsiella pneumoniae bloodstream isolates and frequency of OXA-48, NDM, KPC, VIM, IMP types of carbapenemases in the carbapenem-resistant groups. J Chemother 2021; 1–6. 10.1080/1120009X.2021.1963618. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

