Efficacy and Safety of Roluperidone for the Treatment of Negative Symptoms of Schizophrenia
- PMID: 35211743
- PMCID: PMC9077422
- DOI: 10.1093/schbul/sbac013
Efficacy and Safety of Roluperidone for the Treatment of Negative Symptoms of Schizophrenia
Abstract
Background: This is a placebo-controlled multi-national trial of roluperidone, a compound with antagonist properties for 5-HT2A, sigma2, and α1A-adrenergic receptors, targeting negative symptoms in patients with schizophrenia. This trial follows a previous trial that demonstrated roluperidone superiority over placebo in a similar patient population.
Methods: Roluperidone 32 mg/day, roluperidone 64 mg/day, or placebo was administered for 12 weeks to 513 patients with schizophrenia with moderate to severe negative symptoms. The primary endpoint was the PANSS-derived Negative Symptom Factor Score (NSFS) and the key secondary endpoint was Personal and Social Performance scale (PSP) total score.
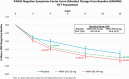
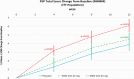
Results: NSFS scores were lower (improved) for roluperidone 64 mg compared to placebo and marginally missing statistical significance for the intent-to-treat (ITT) analysis data set (P ≤ .064), but reached nominal significance (P ≤ .044) for the modified-ITT (m-ITT) data set. Changes in PSP total score were statistically significantly better on roluperidone 64 mg compared to placebo for both ITT and m-ITT (P ≤ .021 and P ≤ .017, respectively).
Conclusions: Results of this trial confirm the potential of roluperidone as a treatment of negative symptoms and improving everyday functioning in patients with schizophrenia. Study registration: Eudra-CT: 2017-003333-29; NCT03397134.
Keywords: negative symptoms; schizophrenia; treatment.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Maryland Psychiatric Research Center.
Figures
Similar articles
-
Long-term effects of Roluperidone on negative symptoms of schizophrenia.Schizophr Res. 2023 May;255:9-13. doi: 10.1016/j.schres.2023.03.028. Epub 2023 Mar 16. Schizophr Res. 2023. PMID: 36933291
-
Effects of Roluperidone (MIN-101) on two dimensions of the negative symptoms factor score: Reduced emotional experience and reduced emotional expression.Schizophr Res. 2020 Jan;215:352-356. doi: 10.1016/j.schres.2019.08.029. Epub 2019 Sep 2. Schizophr Res. 2020. PMID: 31488314 Clinical Trial.
-
Network intervention analysis indicates that roluperidone achieves its effect on negative symptoms of schizophrenia by targeting avolition.Eur Neuropsychopharmacol. 2024 Oct;87:18-23. doi: 10.1016/j.euroneuro.2024.07.005. Epub 2024 Jul 17. Eur Neuropsychopharmacol. 2024. PMID: 39024856 Clinical Trial.
-
Aripiprazole: a review of its use in schizophrenia and schizoaffective disorder.Drugs. 2004;64(15):1715-36. doi: 10.2165/00003495-200464150-00010. Drugs. 2004. PMID: 15257633 Review.
-
Paliperidone extended release: a review of its use in the management of schizophrenia.Drugs. 2010 Jul 9;70(10):1295-317. doi: 10.2165/11204840-000000000-00000. Drugs. 2010. PMID: 20568835 Review.
Cited by
-
New Developments in the Treatment of Schizophrenia: An Expert Roundtable.Int J Neuropsychopharmacol. 2023 May 31;26(5):322-330. doi: 10.1093/ijnp/pyad011. Int J Neuropsychopharmacol. 2023. PMID: 36932673 Free PMC article.
-
Management of cognitive and negative symptoms in schizophrenia.Ment Health Clin. 2022 Nov 3;12(5):282-299. doi: 10.9740/mhc.2022.10.282. eCollection 2022 Oct. Ment Health Clin. 2022. PMID: 36405508 Free PMC article.
-
Corticolimbic circuitry as a druggable target in schizophrenia spectrum disorders: a narrative review.Transl Psychiatry. 2025 Jan 24;15(1):21. doi: 10.1038/s41398-024-03221-2. Transl Psychiatry. 2025. PMID: 39856031 Free PMC article. Review.
-
New Agents in the Treatment of Psychiatric Disorders: What Innovations and in What Areas of Psychopathology?Pharmaceuticals (Basel). 2025 Apr 30;18(5):665. doi: 10.3390/ph18050665. Pharmaceuticals (Basel). 2025. PMID: 40430486 Free PMC article. Review.
-
Turning the Spotlight on Apathy: Identification and Treatment in Schizophrenia Spectrum Disorders.Schizophr Bull. 2023 Sep 7;49(5):1099-1104. doi: 10.1093/schbul/sbad070. Schizophr Bull. 2023. PMID: 37193675 Free PMC article.
References
-
- Galderisi S, Mucci A, Buchanan RW, Arango C. Negative symptoms of schizophrenia: new developments and unanswered research questions. Lancet Psychiatry. 2018;5:664–677. - PubMed
-
- Szczypiński JJ, Gola M. Dopamine dysregulation hypothesis: the common basis for motivational anhedonia in major depressive disorder and schizophrenia? Rev Neurosci. 2018;29:727–744. - PubMed
-
- Millan MJ, Fone K, Steckler T, Horan WP. Negative symptoms of schizophrenia: clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur Neuropsychopharmacol. 2014;24:645–692. - PubMed
-
- Kirkpatrick B. Developing concepts in negative symptoms: primary vs secondary and apathy vs expression. J Clin Psychiatry. 2014;75:30–37. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous