Protein expression of transmembrane protease serine 4 in the gastrointestinal tract and in healthy, cancer, and SARS‑CoV‑2 infected lungs
- PMID: 35211765
- PMCID: PMC8908323
- DOI: 10.3892/mmr.2022.12654
Protein expression of transmembrane protease serine 4 in the gastrointestinal tract and in healthy, cancer, and SARS‑CoV‑2 infected lungs
Abstract
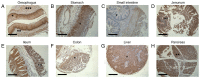
In addition to the angiotensin‑converting enzyme 2 (ACE2), a number of host cell entry mediators have been identified for severe acute respiratory syndrome coronavirus‑2 (SARS‑CoV‑2), including transmembrane protease serine 4 (TMPRSS4). The authors have recently demonstrated the upregulation of TMPRSS4 in 11 different cancers, as well as its specific expression within the central nervous system using in silico tools. The present study aimed to expand the initial observations and, using immunohistochemistry, TMPRSS4 protein expression in the gastrointestinal (GI) tract and lungs was further mapped. Immunohistochemistry was performed on tissue arrays and lung tissues of patients with non‑small cell lung cancer with concurrent coronavirus disease 2019 (COVID‑19) infection using TMPRSS4 antibody. The results revealed that TMPRSS4 was abundantly expressed in the oesophagus, stomach, small intestine, jejunum, ileum, colon, liver and pancreas. Moreover, the extensive TMPRSS4 protein expression in the lungs of a deceased patient with COVID‑19 with chronic obstructive pulmonary disease and bronchial carcinoma, as well in the adjacent normal tissue, was demonstrated for the first time, at least to the best of our knowledge. On the whole, the immunohistochemistry data of the present study suggest that TMPRSS4 may be implicated in the broader (pulmonary and extra‑pulmonary) COVID‑19 symptomatology; thus, it may be responsible for the tropism of this coronavirus both in the GI tract and lungs.
Keywords: coronavirus disease 2019; gastrointestinal tract; lung; severe acute respiratory syndrome coronavirus‑2; transmembrane protease serine 4; tropism.
Conflict of interest statement
DAS is the Editor-in-Chief for the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article. The other authors declare that they have no competing interests.
Figures


Similar articles
-
COVID‑19 and SARS‑CoV‑2 host cell entry mediators: Expression profiling of TMRSS4 in health and disease.Int J Mol Med. 2021 Apr;47(4):64. doi: 10.3892/ijmm.2021.4897. Epub 2021 Mar 2. Int J Mol Med. 2021. PMID: 33649798 Free PMC article.
-
Host cell entry mediators implicated in the cellular tropism of SARS‑CoV‑2, the pathophysiology of COVID‑19 and the identification of microRNAs that can modulate the expression of these mediators (Review).Int J Mol Med. 2022 Feb;49(2):20. doi: 10.3892/ijmm.2021.5075. Epub 2021 Dec 22. Int J Mol Med. 2022. PMID: 34935057 Free PMC article. Review.
-
Expression of ACE2 and TMPRSS2 Proteins in the Upper and Lower Aerodigestive Tracts of Rats: Implications on COVID 19 Infections.Laryngoscope. 2021 Mar;131(3):E932-E939. doi: 10.1002/lary.29132. Epub 2020 Oct 19. Laryngoscope. 2021. PMID: 32940922
-
Characteristics of Angiotensin I-converting enzyme 2, type II transmembrane serine protease 2 and 4 in tree shrew indicate it as a potential animal model for SARS-CoV-2 infection.Bioengineered. 2021 Dec;12(1):2836-2850. doi: 10.1080/21655979.2021.1940072. Bioengineered. 2021. PMID: 34227905 Free PMC article.
-
Interactions of renin-angiotensin system and COVID-19: the importance of daily rhythms in ACE2, ADAM17 and TMPRSS2 expression.Physiol Res. 2021 Dec 16;70(S2):S177-S194. doi: 10.33549/physiolres.934754. Physiol Res. 2021. PMID: 34913351 Free PMC article. Review.
Cited by
-
The immune mechanism of the nasal epithelium in COVID-19-related olfactory dysfunction.Front Immunol. 2023 Jul 17;14:1045009. doi: 10.3389/fimmu.2023.1045009. eCollection 2023. Front Immunol. 2023. PMID: 37529051 Free PMC article. Review.
-
Differential Regulation of Genes by the Glucogenic Hormone Asprosin in Ovarian Cancer.J Clin Med. 2022 Oct 8;11(19):5942. doi: 10.3390/jcm11195942. J Clin Med. 2022. PMID: 36233808 Free PMC article.
-
Pan-Cancer Analysis of the COVID-19 Causal Gene SLC6A20.ACS Omega. 2023 Mar 31;8(14):13153-13161. doi: 10.1021/acsomega.3c00407. eCollection 2023 Apr 11. ACS Omega. 2023. PMID: 37041751 Free PMC article.
References
-
- Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

