Pd(II)-Catalyzed Enantioselective C(sp3)-H Arylation of Cyclopropanes and Cyclobutanes Guided by Tertiary Alkylamines
- PMID: 35212219
- PMCID: PMC9097487
- DOI: 10.1021/jacs.1c11921
Pd(II)-Catalyzed Enantioselective C(sp3)-H Arylation of Cyclopropanes and Cyclobutanes Guided by Tertiary Alkylamines
Abstract
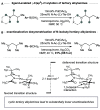
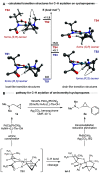
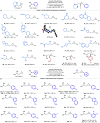
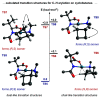
Strained aminomethyl-cycloalkanes are a recurrent scaffold in medicinal chemistry due to their unique structural features that give rise to a range of biological properties. Here, we report a palladium-catalyzed enantioselective C(sp3)-H arylation of aminomethyl-cyclopropanes and -cyclobutanes with aryl boronic acids. A range of native tertiary alkylamine groups are able to direct C-H cleavage and forge carbon-aryl bonds on the strained cycloalkanes framework as single diastereomers and with excellent enantiomeric ratios. Central to the success of this strategy is the use of a simple N-acetyl amino acid ligand, which not only controls the enantioselectivity but also promotes γ-C-H activation of over other pathways. Computational analysis of the cyclopalladation step provides an understanding of how enantioselective C-H cleavage occurs and revealed distinct transition structures to our previous work on enantioselective desymmetrization of N-isobutyl tertiary alkylamines. This straightforward and operationally simple method simplifies the construction of functionalized aminomethyl-strained cycloalkanes, which we believe will find widespread use in academic and industrial settings relating to the synthesis of biologically active small molecules.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Auxiliary-enabled Pd-catalyzed direct arylation of methylene C(sp3)-H bond of cyclopropanes: highly diastereoselective assembling of di- and trisubstituted cyclopropanecarboxamides.Org Lett. 2013 Jul 5;15(13):3238-41. doi: 10.1021/ol4012212. Epub 2013 Jun 17. Org Lett. 2013. PMID: 23772812
-
PdII -Catalyzed Enantioselective C(sp3 )-H Arylation of Cyclobutyl Ketones Using a Chiral Transient Directing Group.Angew Chem Int Ed Engl. 2020 Jun 8;59(24):9594-9600. doi: 10.1002/anie.202000532. Epub 2020 Apr 1. Angew Chem Int Ed Engl. 2020. PMID: 32155313 Free PMC article.
-
Palladium-catalyzed arylation of cyclopropanes via directing group-mediated C(sp3)-H bond activation to construct quaternary carbon centers: synthesis of cis- and trans-1,1,2-trisubstituted chiral cyclopropanes.Org Lett. 2013 Dec 20;15(24):6202-5. doi: 10.1021/ol4030452. Epub 2013 Nov 26. Org Lett. 2013. PMID: 24279330
-
Ring contraction in synthesis of functionalized carbocycles.Chem Soc Rev. 2022 Oct 17;51(20):8652-8675. doi: 10.1039/d1cs01080h. Chem Soc Rev. 2022. PMID: 36172989 Review.
-
Cyclobutanes in Small-Molecule Drug Candidates.ChemMedChem. 2022 May 4;17(9):e202200020. doi: 10.1002/cmdc.202200020. Epub 2022 Mar 29. ChemMedChem. 2022. PMID: 35263505 Free PMC article. Review.
Cited by
-
Synthesis of Polycyclic Imidazoles via α-C-H/N-H Annulation of Alicyclic Amines.Synthesis (Stuttg). 2023 Aug;55(15):2343-2352. doi: 10.1055/a-2022-1511. Epub 2023 Apr 13. Synthesis (Stuttg). 2023. PMID: 38314182 Free PMC article.
-
Asymmetric intermolecular allylic C-H amination of alkenes with aliphatic amines.Science. 2022 Dec 16;378(6625):1207-1213. doi: 10.1126/science.abq1274. Epub 2022 Dec 15. Science. 2022. PMID: 36520916 Free PMC article.
-
Asymmetric synthesis of atropisomers featuring cyclobutane boronic esters facilitated by ring-strained B-ate complexes.Nat Commun. 2024 Dec 30;15(1):10810. doi: 10.1038/s41467-024-55161-6. Nat Commun. 2024. PMID: 39738011 Free PMC article.
-
Metal-catalysed C-C bond formation at cyclopropanes.Nat Rev Chem. 2023 Aug;7(8):548-560. doi: 10.1038/s41570-023-00499-6. Epub 2023 May 22. Nat Rev Chem. 2023. PMID: 37217564 Review.
-
Transition-Metal-Catalyzed C-H Bond Activation for the Formation of C-C Bonds in Complex Molecules.Chem Rev. 2023 Jun 28;123(12):7692-7760. doi: 10.1021/acs.chemrev.2c00888. Epub 2023 May 10. Chem Rev. 2023. PMID: 37163671 Free PMC article. Review.
References
-
- Talele T. T. The “Cyclopropyl Fragment” is a Versatile Player that Frequently Appears in Precilinal/Clinical Drug Molecules. J. Med. Chem. 2016, 59, 8712–8756. 10.1021/acs.jmedchem.6b00472. - DOI - PubMed
- Bauer M. R.; Di Fruscia P.; Lucas S. C. C.; Michaelides I. N.; Nelson J. E.; Storer R. I.; Whitehurst B. C. Put a ring on it: application of small aliphatic rings in medicinal chemistry. RSC Med. Chem. 2021, 12, 448–471. 10.1039/D0MD00370K. - DOI - PMC - PubMed
-
- Hsin L.-W.; Chang L.-T.; Rothman R. B.; Dersch C. M.; Fishback J. A.; Matsumoto R. R. Synthesis and Opioid Activity of Enantiomeric N-Substituted 2,3,4,4a,5,6,7,7a-Octahydro-1H-benzofuro[3,2-e]isoquinolines. J. Med. Chem. 2010, 53, 1392–1396. 10.1021/jm901503e. - DOI - PubMed
- Flick A. C.; Ding H. X.; Leverett C. A.; Fink S. J.; O’Donnell C. J. Synthetic Approaches to New Drugs Approved During 2016. J. Med. Chem. 2018, 61, 7004–7031. 10.1021/acs.jmedchem.8b00260. - DOI - PubMed
- Domon Y.; Arakawa N.; Inoue T.; Matsuda F.; Takahashi M.; Yamamura N.; Kai K.; Kitano Y. Binding Characteristics and Analgesic Effects of Mirogabalin, a Novel Ligand for the α2δSubunit of Voltage-Gated Calcium Channels. J. Pharmacol. Exp. Ther. 2018, 365, 573–582. 10.1124/jpet.117.247551. - DOI - PubMed
- Flick A. C.; Leverett C. A.; Ding H. X.; McInturff E.; Fink S. J.; Mahapatra S.; Carney D. W.; Lindsey E. A.; DeForest J. C.; France S. P.; Berritt S.; Bigi-Botterill S. V.; Gibson T. S.; Liu Y.; O’Donnell C. J. Synthetic Approaches to the New Drugs Approved during 2019. J. Med. Chem. 2021, 64, 3604–3657. 10.1021/acs.jmedchem.1c00208. - DOI - PubMed
-
-
For selected reviews of cyclopropanation reactions, see:
- Davies H. M. L.; Antoulinakis E. G. Intermolecular Metal-Catalyzed Carbenoid Cyclopropanations. Organic Reactions 2001, 57, 1–326. 10.1002/0471264180.or057.01. - DOI
- Bartoli G.; Bencivenni G.; Dalpozzo R. Asymmetric cyclopropanation reactions. Synthesis 2014, 46, 979–1029. 10.1055/s-0033-1340838. - DOI
- Ebner C.; Carreira E. M. Cyclopropanation Strategies in Recent Total Syntheses. Chem. Rev. 2017, 117, 11651–11679. 10.1021/acs.chemrev.6b00798. - DOI - PubMed
- Wu W.; Lin Z.; Jiang H. Recent Advances in the Synthesis of Cyclopropanes. Org. Biomol. Chem. 2018, 16, 7315–7329. 10.1039/C8OB01187G. - DOI - PubMed
- Mato M.; Franchino A.; García-Morales C.; Echavarren A. M. Gold-Catalyzed Synthesis of Small Rings. Chem. Rev. 2021, 121, 8613–8684. 10.1021/acs.chemrev.0c00697. - DOI - PMC - PubMed
-
For selected recent reviews on cyclobutane synthesis, see:
- Poplata S.; Troster A.; Zou Y.-Q.; Bach T. Recent advances in the synthesis of cyclobtanes by olefin [2 + 2] cycloaddition. Chem. Rev. 2016, 116, 9748–9815. 10.1021/acs.chemrev.5b00723. - DOI - PMC - PubMed
- Li J.; Gao K.; Bian M.; Ding H. Recent advances in the total synthesis of cyclobutane containing natural products. Org. Chem. Front. 2020, 7, 136–154. 10.1039/C9QO01178A. - DOI
-
-
- Wasa M.; Engle K. M.; Lin D. W.; Yoo E. J.; Yu J.-Q. Pd(II)-catalyzed Enantioselective C–H Activation of Cyclopropanes. J. Am. Chem. Soc. 2011, 133, 19598–19601. 10.1021/ja207607s. - DOI - PMC - PubMed
- Jerhaoui S.; Djukic J.-P.; Wencel-Delord J.; Colobert F. Asymmetric, Nearly Barrierless C(sp3)–H Activation Promoted by Easily-Accessible N-Protected Aminosulfoxides as New Chiral Ligands. ACS Catal. 2019, 9, 2532–2542. 10.1021/acscatal.8b04946. - DOI
- Chan K. S. L.; Fu H.-Y.; Yu J.-Q. Palladium(II)-Catalyzed Highly Enantioselective C–H Arylation of Cyclopropylmethyl-amines. J. Am. Chem. Soc. 2015, 137, 2042–2046. 10.1021/ja512529e. - DOI - PMC - PubMed
- Shen P.-X.; Hu L.; Shao Q.; Hong K.; Yu J.-Q. Pd(II)-Catalyzed Enantioselective C(sp3)–H Arylation of Free Carboxylic Acids. J. Am. Chem. Soc. 2018, 140, 6545–6549. 10.1021/jacs.8b03509. - DOI - PMC - PubMed
- Zhuang Z.; Yu J.-Q. Pd(II)-Catalyzed Enantioselective γ-C(sp3)-H Functionalizations of Free Cyclo-propylmethylamines. J. Am. Chem. Soc. 2020, 142, 12015–12019. 10.1021/jacs.0c04801. - DOI - PMC - PubMed
-
- Saget T.; Cramer N. Palladium(0)-Catalyzed Enantioselective C–H Arylation of Cyclopropanes: Efficient Access to Functionalized Tetrahydroquinolines. Angew. Chem., Int. Ed. 2012, 51, 12842–12845. 10.1002/anie.201207959. - DOI - PubMed
- Pedroni J.; Saget T.; Donets P. A.; Cramer N. Enantioselective Palladium(0)-Catalyzed Intramolecular Cyclo-propane Functionalization: Access to Dihydroquinolones. Chem. Sci. 2015, 6, 5164–5171. 10.1039/C5SC01909E. - DOI - PMC - PubMed
- Pedroni J.; Cramer N. Chiral γ–Lactams by Enantioselective Palladium(0)-Catalyzed Cyclo-propane Functionalizations. Angew. Chem., Int. Ed. 2015, 54, 11826–11829. 10.1002/anie.201505916. - DOI - PubMed
- Mayer C.; Ladd C. L.; Charette A. B. Utilization of BozPhos as an Effective Ligand in Enantioselective C–H Functionalization of Cyclopropanes: Synthesis of Dihydro-isoquinolones and Dihydroquinolones. Org. Lett. 2019, 21, 2639–2644. 10.1021/acs.orglett.9b00627. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

